Patients with advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma face a high symptom burden, including pain, dysphagia, and appetite loss, which significantly impair health-related quality of life (HRQoL). While first-line combination chemotherapy remains the standard of care, there is a growing emphasis on balancing survival benefits with maintenance of HRQoL.
The phase 3 KEYNOTE-859 trial (NCT03675737) previously demonstrated that pembrolizumab plus chemotherapy significantly improved overall survival (OS), progression-free survival (PFS), and objective response rate (ORR)compared with placebo plus chemotherapy. In this report, prespecified exploratory patient-reported outcomes (PROs) assess the impact of pembrolizumab on HRQoL.
Trial Design and Endpoints
KEYNOTE-859 was a global, randomized, double-blind phase 3 study enrolling 1,579 patients with untreated, locally advanced unresectable or metastatic HER2-negative gastric or GEJ adenocarcinoma. Patients were randomized 1:1 to receive:
- Pembrolizumab 200 mg IV Q3W + chemotherapy (cisplatin/5-FU or CAPOX) for up to 35 cycles
- Placebo + chemotherapy
Randomization was stratified by geographic region, PD-L1 combined positive score (CPS <1 vs ≥1), and chemotherapy regimen.
HRQoL Assessments
HRQoL was evaluated using validated instruments:
- EORTC QLQ-C30 (global health status, physical and role functioning, symptom scales)
- EORTC QLQ-STO22 (gastric cancer–specific symptoms, including pain)
- EQ-5D-5L VAS (overall health status)
Primary PRO endpoints included least squares mean (LSM) change from baseline to week 18 and time to deterioration (TTD), defined as ≥10-point worsening from baseline confirmed at the next visit. Analyses were conducted in the full analysis set (FAS) and stratified by PD-L1 CPS ≥1 and ≥10.
KEYNOTE-859 Trial Results
Results of this prespecified HRQoL analysis were published in the European Journal of Oncology (EJO) in September 2025. Among the 1,531 patients in the PRO FAS population, completion and compliance rates exceeded 95% at baseline and >65% at week 18.
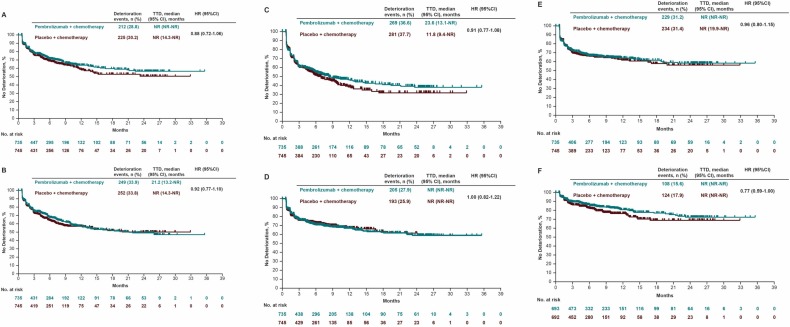
QLQ-C30 Global Health Status/Quality of Life (GHS/QoL)
- LSM change from baseline to week 18:
- Pembrolizumab + chemo: +0.40 (95% CI, –1.40 to 2.19)
- Placebo + chemo: –0.77 (95% CI, –2.56 to 1.01)
- LSM difference: +1.17 (P = .33) → No significant difference
QLQ-STO22 Pain Scale
- LSM change favored pembrolizumab: –8.19 vs –5.61 (LSM difference –2.58; P = .0189)
- TTD also favored pembrolizumab: HR 0.77 (95% CI, 0.59–1.00)
EQ-5D-5L VAS
- No significant between-group differences were observed (LSM difference +1.05; P = .27)
Subgroup analyses by PD-L1 CPS ≥1 and ≥10 populations demonstrated results consistent with the overall population, with numerically longer TTD and improved HRQoL trends in the CPS ≥10 subgroup.
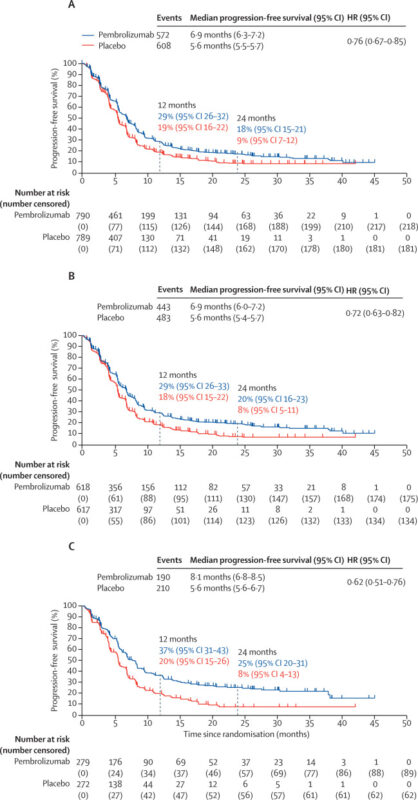
The primary results of the KEYNOTE-859 trial were published in The Lancet Oncology in 2023. Between November 2018 and June 2021, 1,579 patients with previously untreated HER2-negative advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma were randomized to receive pembrolizumab plus chemotherapy (n=790) or placebo plus chemotherapy (n=789).
At a median follow-up of 31 months, pembrolizumab plus chemotherapy significantly improved outcomes:
- Overall survival (primary endpoint): 12.9 vs 11.5 months (HR 0.78; 95% CI 0.70–0.87; p<0.0001)
- PD-L1 CPS ≥1: 13.0 vs 11.4 months (HR 0.74; 95% CI 0.65–0.84; p<0.0001)
- PD-L1 CPS ≥10: 15.7 vs 11.8 months (HR 0.65; 95% CI 0.53–0.79; p<0.0001)
Progression-free survival and objective response rates were also improved, consistent across subgroups.
Safety was manageable: the most common grade 3–5 adverse events were anemia (12% vs 10%) and decreased neutrophil count (10% vs 8%). Serious treatment-related events occurred in 23% vs 19%, and treatment-related deaths in 1% vs 2% of patients. No new safety signals were identified.
Conclusion
Pembrolizumab plus chemotherapy maintained HRQoL during first-line treatment for advanced HER2-negative gastric/GEJ adenocarcinoma, with a significant improvement in pain scores versus placebo. These findings complement previously reported survival benefits and reinforce pembrolizumab plus chemotherapy as a favorable first-line option for this patient population, including those with PD-L1 CPS ≥1 or CPS ≥10 tumors.
Clinical takeaway – KEYNOTE-859 supports pembrolizumab plus chemotherapy as a regimen that not only prolongs survival but also maintains — and in some domains improves — patient-reported quality of life, addressing both clinical outcomes and patient well-being.

Find more information about Top Esophageal and Gastric Cancer Trials to Watch at ESMO 2025 on OncoDaily.