FDA granted traditional approval on march 19, 2025 for pembrolizumab (Keytruda, Merck) in combination with trastuzumab and fluoropyrimidine- and platinum-based chemotherapy as a first-line treatment for adults with locally advanced unresectable or metastatic HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma, whose tumors have PD-L1 expression (CPS ≥1).
Pembrolizumab previously received accelerated approval for this indication on May 5, 2021, based on interim analysis of the trial described below.

Gastric and gastroesophageal junction adenocarcinomas
Gastric and gastroesophageal junction (GEJ) adenocarcinomas are aggressive malignancies with significant global incidence and mortality. Gastric cancer ranks among the leading causes of cancer-related deaths worldwide, with GEJ adenocarcinomas demonstrating increasing prevalence, particularly in Western populations. These cancers are often diagnosed at an advanced stage, making early detection and optimized treatment strategies critical for improving patient outcomes.
The pathogenesis of gastric and GEJ adenocarcinomas is multifactorial, involving genetic, environmental, and microbial factors. Advances in molecular profiling have enabled a deeper understanding of tumor heterogeneity, leading to more precise diagnostic and therapeutic strategies. Additionally, shifts in epidemiological trends, including decreasing distal gastric cancer rates and rising GEJ adenocarcinomas, highlight the need for targeted screening and prevention efforts.
Despite improvements in treatment modalities, prognosis remains poor for advanced cases, underscoring the importance of continued research into novel therapeutic options, early detection methods, and personalized medicine approaches.
Current Treatment Strategies
For patients with unresectable locally advanced, recurrent, or metastatic Disease (where local therapy is not indicated) the standard treatment was chemotherapy with Fluoropyrimidine (fluorouracil or capecitabine), oxaliplatin, and trastuzumab. In May 2025 Pembrolizumab was granted accelerated approval and was added to the standard of treatment.
What is Pembrolizumab and what is the mechanism of action
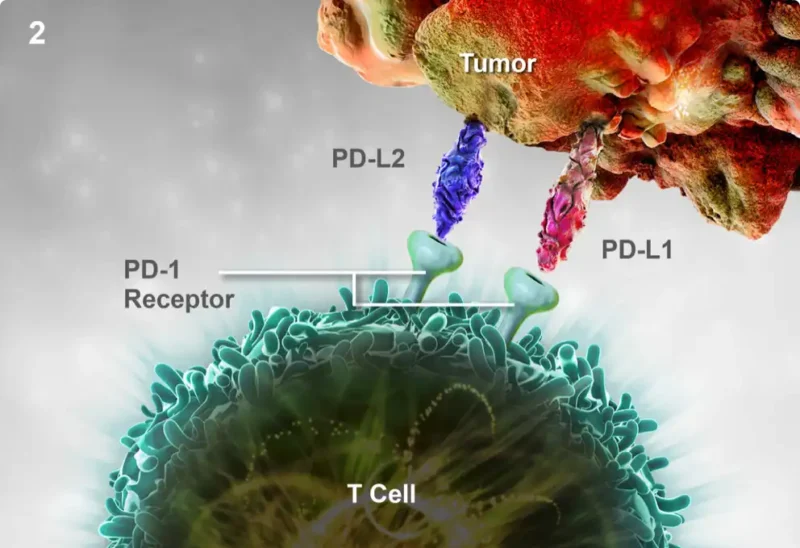
Pembrolizumab is a monoclonal antibody that targets the programmed death-1 (PD-1) receptor, an immune checkpoint expressed on T cells. By binding to PD-1, pembrolizumab blocks its interaction with its ligands, PD-L1 and PD-L2, thereby preventing immune evasion by tumor cells. This restores T-cell activation and enhances the immune response against cancer cells. The clinical efficacy of pembrolizumab is particularly pronounced in tumors with high PD-L1 expression, microsatellite instability-high (MSI-H) status, or mismatch repair deficiency (dMMR), making it a key therapeutic option in gastric and GEJ adenocarcinomas.
KEYNOTE-811 Trial and Pembrolizumab Efficacy
The efficacy of pembrolizumab in HER2-positive advanced gastric and GEJ adenocarcinoma was demonstrated in the KEYNOTE-811 trial (NCT03615326), a multicenter, randomized, double-blind, placebo-controlled study enrolling 698 patients. Of these, 594 (85%) had PD-L1-positive tumors (CPS ≥1). Patients received pembrolizumab 200 mg or placebo in combination with trastuzumab and chemotherapy (fluorouracil plus cisplatin or capecitabine plus oxaliplatin).
Key efficacy outcomes included progression-free survival (PFS) and overall survival (OS), assessed by blinded independent central review (BICR). Pembrolizumab demonstrated significant clinical benefit:
- Median PFS: 10.9 months (95% CI: 8.5, 12.5) vs. 7.3 months (95% CI: 6.8, 8.4) in the placebo arm (HR 0.72 [95% CI: 0.60, 0.87]).
- Median OS: 20.1 months (95% CI: 17.9, 22.9) vs. 15.7 months (95% CI: 13.5, 18.5) in the placebo arm (HR 0.79 [95% CI: 0.66, 0.95]).
- Overall response rate (ORR): 73% (95% CI: 68, 78) vs. 58% (95% CI: 53, 64).
- Median duration of response (DOR): 11.3 months (95% CI: 9.9, 13.7) vs. 9.6 months (95% CI: 7.1, 11.2).
The safety profile of pembrolizumab was consistent with its known adverse event profile. The recommended dose is 200 mg every 3 weeks or 400 mg every 6 weeks in combination with trastuzumab and chemotherapy.
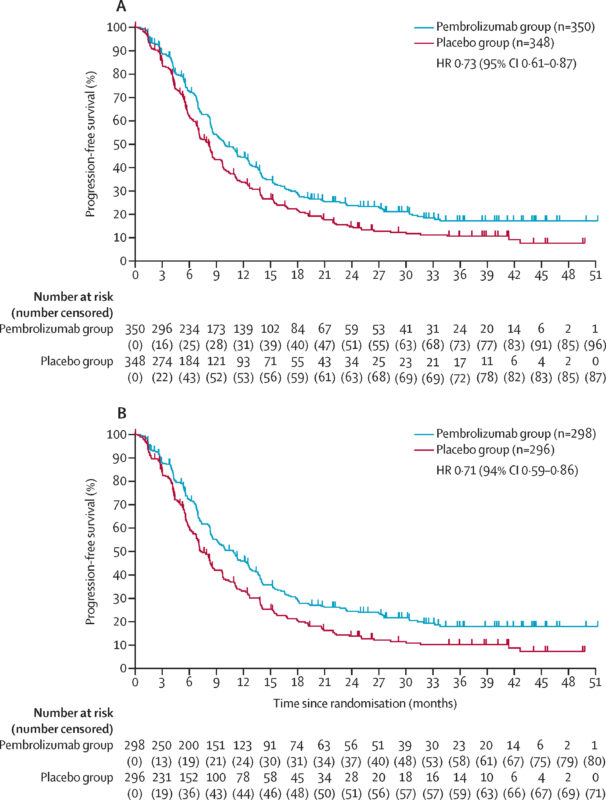
Impact of the KEYNOTE-811 Trial
The KEYNOTE-811 trial marked a significant shift in the management of HER2-positive advanced gastric and GEJ adenocarcinoma by establishing pembrolizumab as a key addition to trastuzumab and chemotherapy. This multicenter, randomized, double-blind, placebo-controlled study enrolled 698 patients, of whom 85% had PD-L1-positive tumors (CPS ≥1). Patients received either pembrolizumab 200 mg or a placebo, both in combination with trastuzumab and chemotherapy (fluorouracil plus cisplatin or capecitabine plus oxaliplatin).
The study demonstrated statistically significant and clinically meaningful improvements in key efficacy endpoints mentioned above.
Clinical Implications
These findings transformed first-line therapy for HER2-positive advanced gastric and GEJ adenocarcinomas, leading to the FDA approval of pembrolizumab in combination with trastuzumab and chemotherapy for patients with PD-L1 CPS ≥1. This study reinforced the importance of biomarker-driven treatment strategies, particularly for PD-L1-expressing tumors, and established checkpoint inhibition as a critical component of first-line therapy.
The recommended pembrolizumab dosing is 200 mg every 3 weeks or 400 mg every 6 weeks in combination with trastuzumab and chemotherapy.
written by Sona Karamyan,MD


