OptiTROP-Lung04 (NCT05870319), a pivotal phase 3 trial presented by Prof. Li Zhang (Guangzhou, China) at the ESMO Congress 2025, evaluated the efficacy and safety of the novel TROP2-directed antibody–drug conjugate (ADC) Sac-TMT versus platinum-based chemotherapy in patients with EGFR-mutated (EGFRm) non–small cell lung cancer (NSCLC) who had progressed after prior EGFR tyrosine kinase inhibitor (TKI) and platinum-based therapy. The study demonstrated statistically significant and clinically meaningful improvements in both progression-free survival (PFS) and overall survival (OS) with Sac-TMT, positioning it as a potential new standard of care for this treatment-resistant population.
Background
Patients with EGFR-mutant NSCLC who experience progression after third-generation EGFR TKIs face limited therapeutic options, and outcomes with standard chemotherapy remain poor. Sac-TMT is a next-generation TROP2-targeting ADC designed using a novel cleavable linker to attach a belotecan-derived topoisomerase I inhibitor payload, enabling potent, tumor-selective cytotoxicity while minimizing systemic toxicity.
Earlier findings (Fang et al., BMJ, 2025) demonstrated that Sac-TMT significantly improved survival compared with docetaxel in patients with EGFRm NSCLC after EGFR TKI and platinum therapy. The OptiTROP-Lung04 trial was therefore designed to validate these results in a head-to-head comparison with platinum doublet chemotherapy in a global phase 3 setting.
Methods
The trial enrolled 376 patients with stage IIIB–IV EGFR-mutant NSCLC who had previously received EGFR TKI and platinum-based chemotherapy. Participants were randomized 1:1 to receive either:
- Sac-TMT 5 mg/kg every 2 weeks
- Chemotherapy: pemetrexed 500 mg/m² + carboplatin (AUC 5) or cisplatin (75 mg/m²) every 3 weeks for 4 cycles, followed by pemetrexed maintenance.
The primary endpoint was PFS assessed by blinded independent review committee (BIRC). Overall survival (OS) was a key secondary endpoint, tested hierarchically. Additional endpoints included objective response rate (ORR), duration of response (DOR), and safety.
Results
At a median follow-up of 18.9 months, Sac-TMT achieved significant and clinically meaningful efficacy improvements versus chemotherapy:
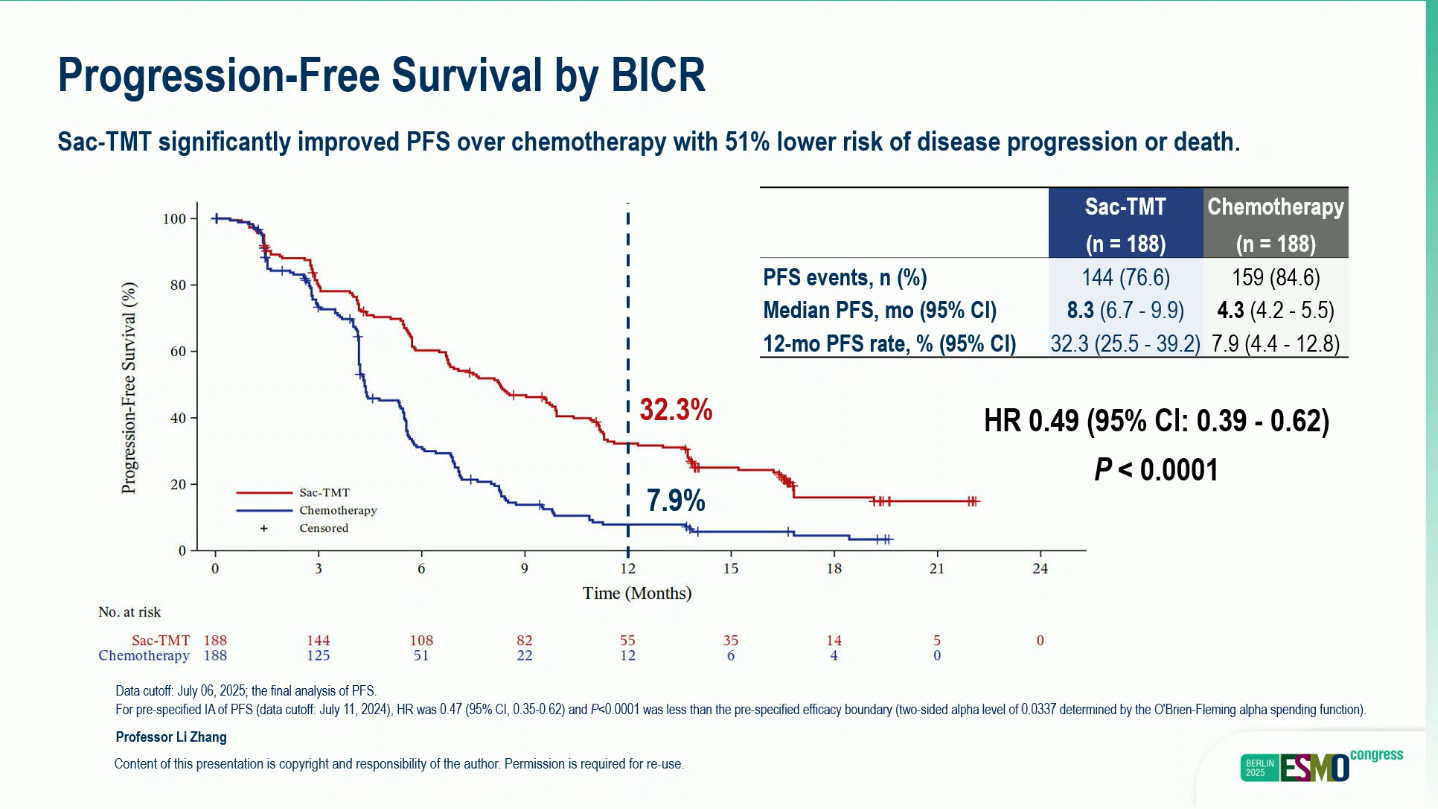
- Median PFS (BIRC): 8.3 months (95% CI, 6.7–9.9) vs 4.3 months (95% CI, 4.2–5.5) (HR 0.49; 95% CI, 0.39–0.62; p < 0.0001)
- 12-month PFS rate: 32.3% vs 7.9%
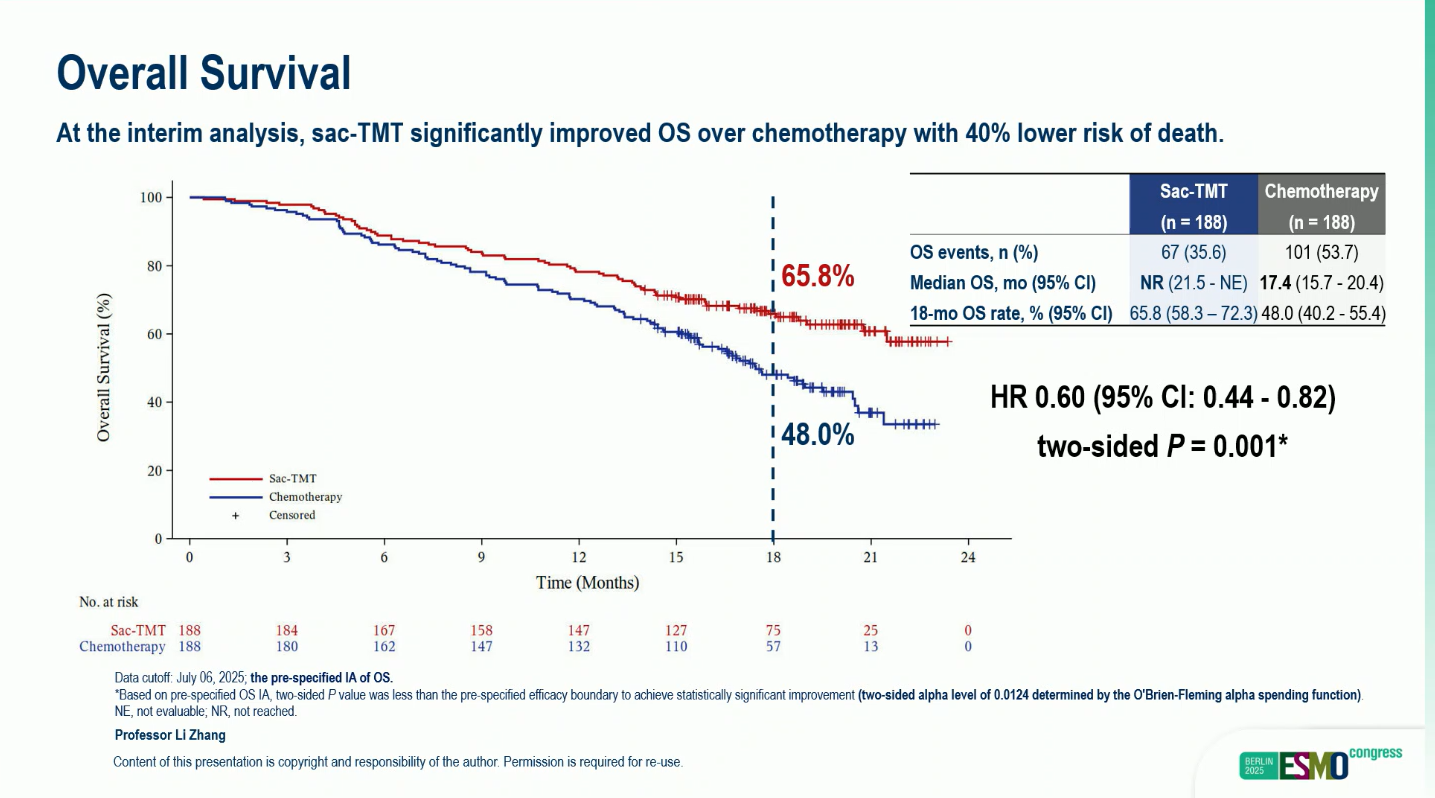
- Median OS: Not reached (95% CI, 21.5–NE) vs 17.4 months (95% CI, 15.7–20.4) (HR 0.60; 95% CI, 0.44–0.82; two-sided p = 0.001)
- Adjusted median OS (censored for subsequent ADC use): HR 0.56 (95% CI, 0.41–0.77; p = 0.0002)
- ORR (BIRC): 60.6% (95% CI, 53.3–67.7) vs 43.1% (95% CI, 35.9–50.5)
- Median DOR (BIRC): 8.3 vs 4.2 months
At data cutoff (July 6, 2025), 21.3% of patients on Sac-TMT remained on treatment compared with only 1.6% on chemotherapy, suggesting durable benefit and disease control.
Safety
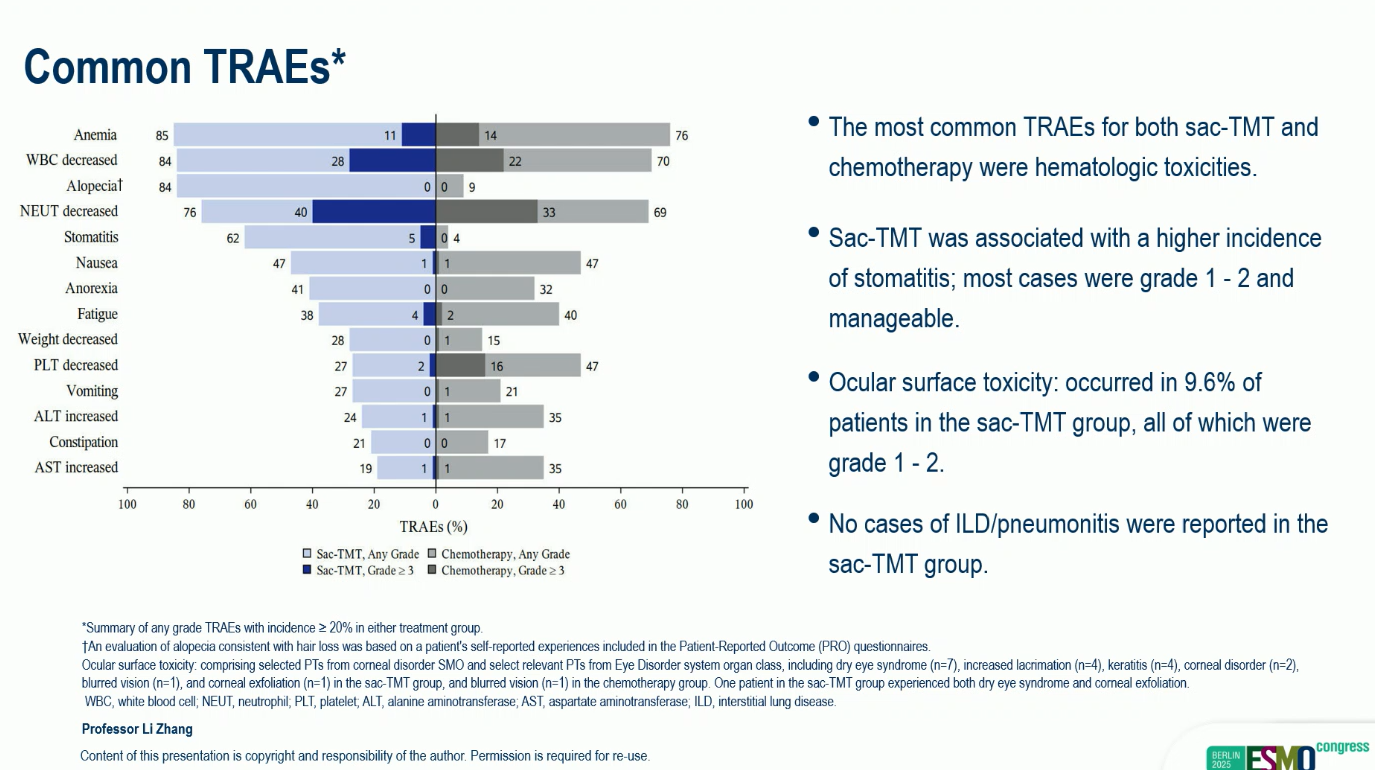
Sac-TMT was well tolerated, with a manageable safety profile and no new safety signals.
- Grade ≥3 treatment-related adverse events (TRAEs): 58% vs 53%
- No drug-related interstitial lung disease (ILD) or pneumonitis was reported in either arm.
- The most frequent adverse events were hematologic and gastrointestinal, consistent with known profiles of ADCs and chemotherapy.
Conclusions
Presented by Prof. Li Zhang, the OptiTROP-Lung04 trial confirmed that Sac-TMT delivers significant and durable PFS and OS benefits over platinum-based chemotherapy in patients with EGFR TKI–resistant NSCLC, marking a new era for TROP2-directed ADC therapy. With robust efficacy and favorable tolerability, Sac-TMT is poised to become a new standard of care in the post-TKI treatment landscape for EGFR-mutated advanced NSCLC.
You can read the full abstract here.


