August 18, 2025 – The European Commission (EC) has granted marketing authorization for OGSIVEO (nirogacestat) as monotherapy for the treatment of adults with progressing desmoid tumors who require systemic therapy.
Developed by SpringWorks Therapeutics, Inc., a healthcare company of Merck KGaA, Darmstadt, Germany, OGSIVEO is now the first and only approved therapy in the European Union for desmoid tumors. This approval marks a significant advance for patients across Europe, bringing a targeted oral treatment option to a rare tumor type that previously had no authorized systemic therapies.
“Desmoid tumors can have a profound impact on people’s lives and are difficult to manage due to their invasive nature and high rates of recurrence. Until now, there have been no approved medicines in Europe,”
said Prof. Bernd Kasper, M.D., Ph.D., University of Heidelberg and principal investigator of the DeFi trial.
“OGSIVEO is a highly innovative therapy with efficacy data demonstrating both meaningful antitumor activity and a significant improvement in desmoid tumor symptoms, including a significant reduction in pain which is the most debilitating symptom reported by patients.”
Lynne Hernandez, Executive Director of the Desmoid Tumor Research Foundation, emphasized the importance of this milestone:
“This approval is a long-awaited advance for desmoid tumor patients, their families and physicians in Europe. It is our hope that patients will benefit from greater awareness of desmoid tumors, faster diagnoses, and better outcomes now that there is an approved treatment.”

LinkedIn photo of Lynne Hernandez
The DeFi Trial: Efficacy and Safety Outcomes
The Phase 3 DeFi trial (NCT03785964) formed the basis of the EC approval. This was a global, multicenter, randomized, double-blind, placebo-controlled study that enrolled 142 adult patients with progressing desmoid tumors. Patients were randomized in a 1:1 ratio to receive nirogacestat 150 mg twice daily or placebo.
Eligibility criteria required documented tumor progression of at least 20% within the prior 12 months, as measured by RECIST v1.1. The primary endpoint of the trial was progression-free survival (PFS) as assessed by blinded independent central review. Key secondary endpoints included objective response rate (ORR), duration of response, reduction in tumor volume, patient-reported outcomes (PROs), and safety and tolerability.
Trial Results
The study met its primary endpoint, showing that nirogacestat reduced the risk of disease progression or death by 71% compared with placebo (hazard ratio 0.29; 95% CI: 0.15–0.55; p < 0.001).
Nirogacestat also demonstrated superior efficacy in secondary measures. The confirmed objective response rate was 41% in the OGSIVEO arm versus 8% with placebo (p < 0.001). Complete responses occurred in 7% of patients treated with OGSIVEO, while no complete responses were reported in the placebo group. Responses occurred more quickly with treatment, with a median time to first response of 5.6 months versus 11.1 months.
Beyond tumor shrinkage, patients reported clinically meaningful improvements in pain reduction, desmoid tumor–specific symptoms, role functioning, and overall quality of life. These improvements were observed early and sustained throughout the trial.
Safety Profile
The safety data were consistent with prior studies of nirogacestat. The most common adverse events included diarrhea, nausea, fatigue, and hypophosphatemia. While the majority of side effects were grade 1–2, grade 3 adverse events were reported in approximately 24% of patients receiving nirogacestat. Discontinuation due to adverse events occurred in 20% of patients in the nirogacestat arm, underscoring the importance of proactive toxicity management.
Find full information about Nirogacestat EC Approval on SpringWorks Therapeutics Official Website.
What is OGSIVEO and How does it work?
Ogsiveo exerts its anticancer effects by selectively inhibiting the gamma‑secretase enzyme complex, which is involved in key cell signaling pathways. Gamma‑secretase normally cleaves transmembrane proteins, such as the Notch receptor, releasing the Notch intracellular domain (NICD). Once inside the nucleus, NICD activates genes that promote cell proliferation and survival. By blocking this cleavage, nirogacestat prevents NICD formation, disrupting Notch signaling and reducing tumor cell growth—an effect particularly significant in desmoid tumors.
In addition, gamma‑secretase also cleaves BCMA (B-cell maturation antigen) on multiple myeloma cells, releasing a soluble fragment that can interfere with BCMA-targeted therapies. Nirogacestat’s inhibition of this process helps maintain BCMA on the cell surface, increases the number of available targets for drugs, and reduces soluble BCMA “decoys,” potentially enhancing the efficacy of BCMA-directed treatments.
The drug is already approved in the United States, where it has become the standard of care systemic therapy for desmoid tumors, and it now holds both FDA and EC approval.
“We would like to extend our gratitude to the patients, families, investigators, and advocacy organizations who helped make this EC approval possible,”
said Danny Bar-Zohar, M.D., CEO of Healthcare and Executive Board Member, Merck KGaA, Darmstadt, Germany.
“OGSIVEO is already established as the standard of care systemic therapy for desmoid tumors in the U.S., and our goal is to bring the same treatment benefits to patients in Europe.”
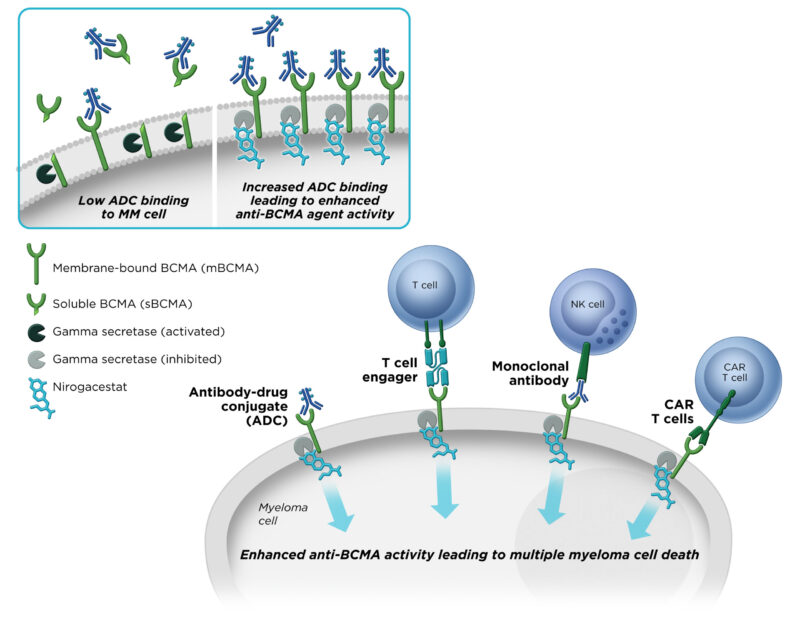
Photo from SpringWorks Therapeutics Official Website
About Desmoid Tumors
Desmoid tumors, also known as aggressive fibromatosis, are rare and locally aggressive soft tissue tumors that can cause severe pain, loss of mobility, functional impairment and disfigurement. While they do not metastasize, they are unpredictable and have recurrence rates as high as 77% after surgery. Because of this, systemic medical therapy has become the recommended first-line treatment approach in most cases.
The disease is typically diagnosed in young adults, most often between ages 20 and 44, and is two to three times more common in women. It is estimated that between 1,300 and 2,300 new cases are diagnosed each year across the EU.
Conclusion
The EC approval of OGSIVEO (nirogacestat) represents a major advance in the care of patients with desmoid tumors in Europe. Backed by strong efficacy data from the DeFi trial, a manageable safety profile, and meaningful improvements in quality of life, OGSIVEO offers patients the first approved systemic therapy for this rare but debilitating condition.
Written by Mariam Khachatryan, MD



