The NO-CUT trial was launched to address one of the most important questions in modern rectal cancer management: whether patients with stage II–III rectal adenocarcinoma who achieve a clinical complete response after total neoadjuvant therapy can safely avoid rectal surgery without compromising long-term oncologic outcomes. As total neoadjuvant therapy (TNT) has become increasingly used for proficient mismatch repair/microsatellite stable (pMMR/MSS) tumors, a growing number of patients reach deep tumor regression, raising the prospect of organ preservation through a structured non-operative management (NOM) approach.
However, although NOM offers substantial quality-of-life advantages by avoiding total mesorectal excision and the morbidity of pelvic surgery, concerns have persisted regarding the risk of distant metastatic relapse when surgery is omitted. The NO-CUT study was designed to prospectively evaluate this balance, combining a standardized TNT regimen with rigorous response assessments and integrating translational tools such as plasma-only liquid biopsy and tumor transcriptomics to refine the biology underlying complete responses.
NO-CUT Design and Endpoints
NO-CUT is a multicentre, investigator-initiated, single-arm, phase 2 trial conducted across four Italian cancer centers. It enrolled adults with untreated, pMMR/MSS stage II–III adenocarcinoma of the lower-to-middle rectum, ECOG 0–1, and no metastases. All participants were candidates for chemoradiotherapy and total mesorectal excision. TNT consisted of four cycles of CAPOX followed by capecitabine-based chemoradiotherapy (50–54 Gy over 5 weeks). Tumor response was assessed through a structured three-step evaluation: after induction CAPOX, 11–12 weeks post-chemoradiotherapy, and—for near-complete responders—an additional MRI 4–5 weeks later. Patients with cCR entered NOM; all others underwent surgery.
Surveillance in NOM included frequent exams, MRI, CT, and endoscopy. The primary endpoint was 30-month distant relapse-free survival (DRFS) in the NOM cohort. Secondary outcomes included local regrowth or relapse, overall survival, colostomy-free survival, clinical complete response rate, and patient-reported quality of life. Exploratory analyses examined ctDNA (Guardant Reveal) and transcriptomic signatures as predictors of response and relapse.

You can also read about Radiotherapy for Rectal Cancer: Types, Success Rate, Side Effects And More on OncoDaily.
Results of NO-CUT
The full results were published in The Lancet Oncology (December 2025). A total of 180 patients began TNT; 179 formed the intention-to-treat population. TNT completion rates were high: 94% completed induction CAPOX and 92% completed chemoradiotherapy. After TNT, 47 patients (26%) achieved a clinical complete response and entered NOM. An additional 10 patients who underwent surgery were found to have pathological complete response, yielding an overall complete response rate of 32%. The multidisciplinary algorithm correctly identified 82% of complete responders for NOM. With a median follow-up of 35 months, DRFS at 30 months was:
- 95% in the NOM cohort, meeting and exceeding the predefined efficacy benchmark
- 74% in surgical patients after TNT
- 74% in the overall population
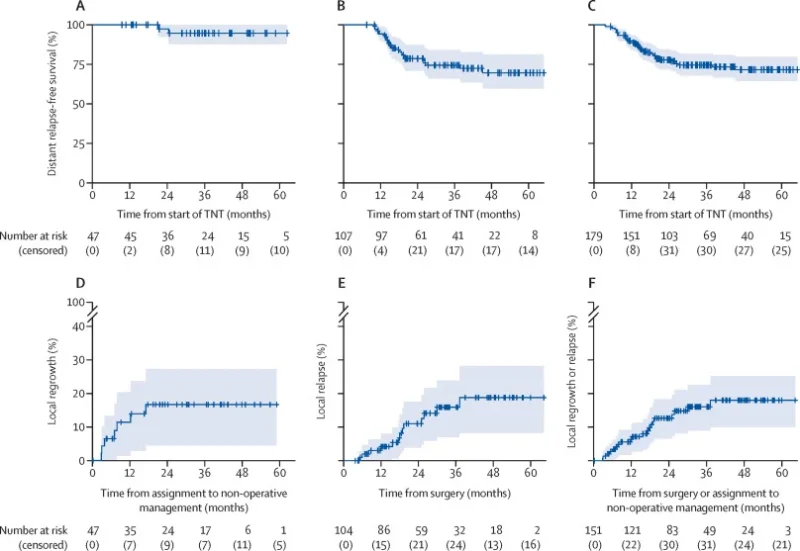
Local regrowth occurred in 7 of 47 patients (15%) in the NOM group, all within two years and all successfully salvaged with curative-intent surgery. Two-year surgery-free survival after NOM was 83%. In the surgery cohort, local relapse risk was 11% at 2 years and 16% at 3 years. Distant progression during or immediately after TNT occurred in about 6% of patients—likely reflecting the higher proportion of cT4 and cN2 disease.
Safety
Grade 3–4 adverse events occurred in 31% of patients, most commonly diarrhea, lymphopenia, and neutropenia (each ~4%). Serious adverse events occurred in 17%. There were no treatment-related deaths. Twelve patients discontinued TNT due to toxicity. ctDNA and translational insights Liquid biopsy was highly informative. At baseline, ctDNA was positive in 95% of patients. After TNT, positivity fell to 24%, but only 8% among patients with cCR. ctDNA was strongly predictive of outcomes:
- Patients in the NOM cohort who remained ctDNA-positive after TNT had significantly worse distant relapse-free and progression-free survival.
- In surgical patients, ctDNA negativity before surgery correlated strongly with pathological complete response, while ctDNA positivity after surgery predicted higher risk of distant relapse, progression, and local recurrence.
- Multivariable models confirmed post-TNT ctDNA negativity as an independent predictor of reduced relapse risk.
Transcriptomic signatures showed trends (higher leukocyte scores in cCR), but no robust genomic classifier emerged. A nomogram integrating clinical features and ctDNA was developed and internally validated.
Conclusion
NO-CUT provides compelling prospective evidence that non-operative management after total neoadjuvant therapy is safe and does not compromise distant disease control in patients with pMMR/MSS stage II–III rectal cancer who achieve clinical complete response. Local regrowths are infrequent, occur early, and are consistently salvageable with surgery. The integration of ctDNA adds an important biological dimension: clearance after TNT aligns with higher response rates and lower risk of relapse, while persistent ctDNA identifies patients needing closer surveillance or altered management.
Together, these findings strengthen NOM as a guideline-supported option for rectal cancer and support a future in which organ preservation strategies are refined by biological markers, response dynamics, and personalized surveillance—moving rectal cancer treatment toward less invasive, high-precision care.