New Paper Published in JCO Oncology Practice on Dec 17, 2024 on HR+ Breast Cancer Treatment Options After Progression on CDK4/6 Inhibitors.

Title: Efficacy of Subsequent Treatments After Disease Progression on CDK4/6 Inhibitors in Patients With Hormone Receptor–Positive Advanced Breast Cancer
Authors: Lis Victoria Ravani, MS, Pedro Calomeni, MS, Maysa Vilbert, MD h, Thiago Madeira, MS, Ming Wang, PhD, Daxuan Deng, MS, Laura Testa, MD, Renata Colombo Bonadio, MD, Ph Katherine Clifton, MD Seth A. Wander, MD, PhD, and Maryam Lustberg, MD, MPH
Background
Hormone receptor–positive and or human epidermal growth factor receptor 2–negative (HR+/HER2–) advanced breast cancer (aBC) still represents a clinical challenge. Cyclin-dependent kinase 4/6 inhibitors (CDK4/6) with endocrine therapy (ET) are the current standard of care for these patients. However, many people progress on the standard treatment and unfortunately there is a lack of optimal post-progression treatment strategies. This study aimed to analyze the efficacy of different therapies following progression on CDK4/6i-based regimens.
Methods
A systematic review and meta-analysis were conducted to assess post-progression treatment outcomes in patients with HR+/HER2– aBC. The review included studies published between 2016 and December 2023, identified through searches of PubMed, Embase, Cochrane, and major conference proceedings. The primary outcomes were progression-free survival (PFS) and overall survival (OS). A pooled analysis of Kaplan-Meier-derived individual patient data was performed to evaluate treatment efficacy.
Study Design
The study included 18 eligible trials comprising a total of 4,899 patients with HR+/HER2– aBC who had experienced progression on CDK4/6i therapy. Key treatment strategies analyzed were:
- Continuing CDK4/6i combined with ET (either the same CDK4/6i or switching to a different one).
- Transitioning to everolimus plus ET.
- ET monotherapy as a control.
Outcomes were compared across these groups using hazard ratios (HRs) for PFS and OS, with statistical significance set at P<.01.
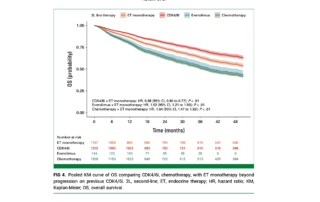
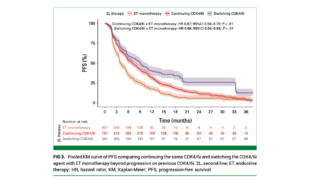
Results
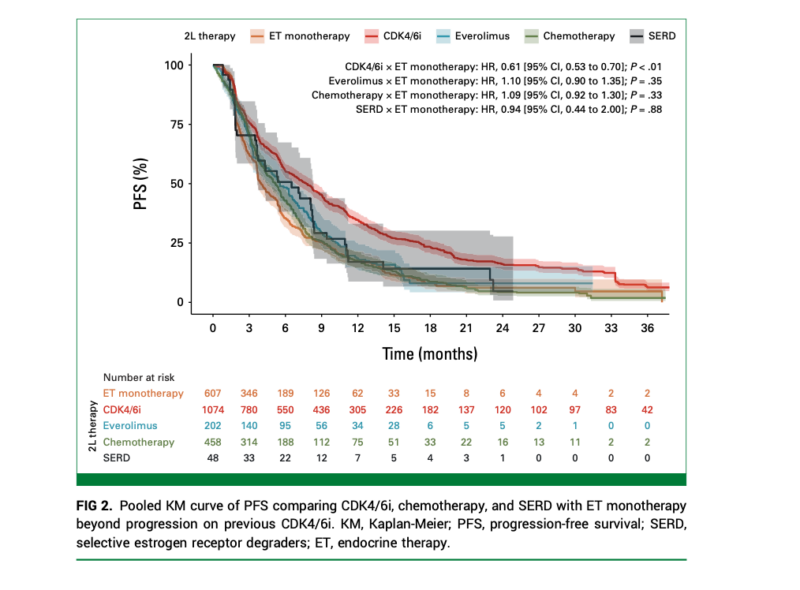
Among the analyzed studies, continuing CDK4/6i therapy with ET after progression provided significant benefits over ET monotherapy:
- Progression-Free Survival (PFS):
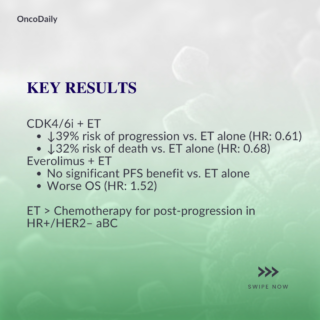
- Continuing CDK4/6i with ET resulted in a 39% reduction in progression risk compared to ET monotherapy (HR, 0.61; 95% CI, 0.53 to 0.70; P < .01).
- Benefits were consistent whether the same CDK4/6i was continued (HR, 0.67; 95% CI, 0.56 to 0.79) or a different CDK4/6i was used (HR, 0.68; 95% CI, 0.54 to 0.85).
- Overall Survival (OS):
- Patients receiving CDK4/6i plus ET experienced a 32% reduction in the risk of death compared to ET monotherapy (HR, 0.68; 95% CI, 0.60 to 0.77; P < .01).
In contrast, transitioning to everolimus plus ET did not provide a significant PFS advantage over ET monotherapy (HR, 1.10; 95% CI, 0.90 to 1.35; P = .35). Moreover, everolimus plus ET was associated with a significantly shorter OS (HR, 1.52; 95% CI, 1.21 to 1.90; P < .01), indicating a potential survival disadvantage.
Key Findings
- Continuation or Switch of CDK4/6i: Maintaining CDK4/6i therapy, whether by continuing the same agent or switching to another, combined with ET, substantially improved both PFS and OS compared to ET monotherapy.
- PFS: 0.61 HR vs. ET monotherapy.
- OS: 0.68 HR vs. ET monotherapy.
- Everolimus Plus ET: Although everolimus combined with ET showed similar PFS to ET monotherapy, OS was shorter, highlighting its limited utility as a post-progression option.
- ET-Based Approaches Preferred: Across all comparisons, ET-based regimens demonstrated greater efficacy than chemotherapy for post-progression treatment in HR+/HER2– aBC, underscoring their continued role as the foundation of sequencing strategies.
Key Takeaway Messages
- Maintaining CDK4/6i therapy in combination with ET is a promising strategy for managing HR+/HER2– aBC after disease progression.
- Switching to everolimus plus ET should be approached with caution due to potential OS disadvantages.
- Current guidelines recommending ET-based therapies over chemotherapy for post-progression sequencing are strongly supported by this pooled analysis.
Conclusion
This study provides strong evidence supporting the use of CDK4/6 inhibitors with endocrine therapy after disease progression on CDK4/6 inhibitors in HR+/HER2– advanced breast cancer. Moving forward, prospective trials are needed to refine post-progression strategies further, ensuring the best outcomes for patients. These findings emphasize the role of CDK4/6 inhibitors, not only as first-line therapy but also as an option for continued use in later lines of treatment, transforming the management for HR+/HER2– advanced breast cancer.
Read the full article on JCO Oncology Practice
Summary by Sona Karamyan, MD