The phase 3 ESOPEC trial compared perioperative FLOT vs. chemoradiotherapy in gastric cancer, revealing improved overall survival (OS) and progression-free survival (PFS) with the FLOT regimen. Published in The New England Journal of Medicine on January 22, 2025, this study provides critical insights into optimizing treatment strategies for resectable esophageal adenocarcinoma.
Authors: J. Hoeppner, T. Brunner, C. Schmoor, P. Bronsert, B. Kulemann, R. Claus, S. Utzolino, J.R. Izbicki, I. Gockel, B. Gerdes, M. Ghadimi, B. Reichert, J.F. Lock, C. Bruns, E. Reitsamer, M. Schmeding, F. Benedix, T. Keck, G. Folprecht, P. Thuss‑Patience, U.P. Neumann, A. Pascher, D. Imhof, S. Daum, T. Strieder, C. Krautz, S. Zimmermann, J. Werner, R. Mahlberg, G. Illerhaus, P. Grimminger, and F. Lordick
Background
Esophageal cancer affects over 510,000 people annually and remains a leading cause of cancer mortality worldwide, with over 445,000 deaths per year. The incidence of esophageal adenocarcinoma and esophagogastric junction tumors continues to rise, particularly in high-income countries.Radical esophagectomy is the curative standard for nonmetastatic resectable esophageal adenocarcinoma, but recurrence rates remain high, with 5-year survival rates rarely exceeding 35%. Two multimodal treatment strategies have shown survival benefits:
- Preoperative chemoradiotherapy (CROSS protocol): Weekly carboplatin and paclitaxel with 41.4 Gy radiotherapy, followed by surgery.
- Perioperative chemotherapy (FLOT regimen): Four cycles of fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) before and after surgery.
The ESOPEC trial was designed to directly compare these two approaches in terms of overall survival.
Methods
The ESOPEC trial was a phase 3, multicenter, randomized controlled trial conducted across 25 centers in Germany. Patients with histologically confirmed resectable esophageal adenocarcinoma (UICC clinical stage cT1 cN+, cT2-4a cN+, or cT2-4a cN0) were randomized 1:1 to receive:
- Perioperative FLOT chemotherapy: Four preoperative and four postoperative cycles (fluorouracil, leucovorin, oxaliplatin, docetaxel).
- Preoperative chemoradiotherapy (CROSS regimen): Weekly carboplatin and paclitaxel with 41.4 Gy radiotherapy followed by surgery.
Primary endpoint: Overall survival (OS) Secondary endpoints: Progression-free survival (PFS), recurrence rates, surgical outcomes, adverse events.
Results
The ESOPEC trial demonstrated a significant survival advantage with perioperative FLOT chemotherapy compared to preoperative chemoradiotherapy, reinforcing its role as the preferred treatment for resectable esophageal adenocarcinoma.
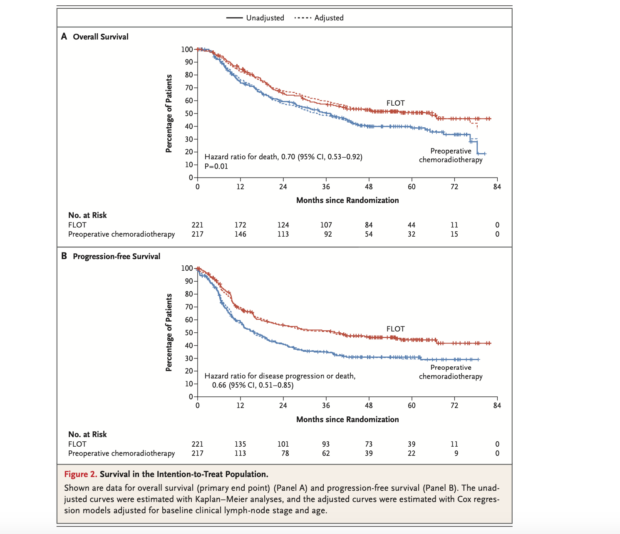
- 3-Year Overall Survival (OS): 57.4% (FLOT) vs. 50.7% (Chemoradiotherapy) (HR=0.70, 95% CI: 0.53-0.92, P=0.01)
- 5-Year Overall Survival (OS): 50.6% (FLOT) vs. 38.7% (Chemoradiotherapy)
- Median Overall Survival of 66 months (FLOT) vs. 37 months (Chemoradiotherapy)
- 3-Year Progression-Free Survival (PFS): 51.6% (FLOT) vs. 35.0% (Chemoradiotherapy) (HR=0.66, 95% CI: 0.51-0.85)
- Distant Metastases were higher in Chemoradiotherapy (71 vs. 45 patients)
- Locoregional Recurrence was higher in FLOT (17 vs. 9 patients)
- R0 Resection Rates were Similar (94.3% vs. 95.0%)
- Pathological Complete Response (pCR): 16.7% (FLOT) vs. 10.1% (Chemoradiotherapy)
- Severe Adverse Events (Grade 3+): 58.0% (FLOT) vs. 50.0% (Chemoradiotherapy)
Key Takeaways
- FLOT significantly improved OS and PFS compared to chemoradiotherapy.
- Higher rates of distant metastases were seen in the chemoradiotherapy group.
- FLOT was associated with more systemic control, while chemoradiotherapy showed better locoregional control.
- No major difference in R0 resection rates or surgical complications.
Conclusion
The trial aimed to determine whether perioperative chemotherapy using the FLOT regimen provides superior overall survival compared to preoperative chemoradiotherapy for patients with resectable esophageal adenocarcinoma. Findings demonstrated a notable survival benefit with FLOT, with a median overall survival of 66 months, compared to 37 months in the chemoradiotherapy group.
Overall, this trial demonstrated that perioperative FLOT chemotherapy significantly improves survival over preoperative chemoradiotherapy in patients with resectable esophageal adenocarcinoma, including those with cN+ lymph-node involvement and more advanced tumor stages (cT3 or cT4), who constituted the majority of participants. However, it remains uncertain whether a de-escalated chemotherapy doublet or a shift to preoperative chemoradiotherapy is preferable for patients unable to tolerate FLOT due to comorbidities or treatment-related adverse effects—an issue that this study was not designed to address.
You can read the full article in the New England Journal of Medicine
Written by Sona Karamyan


