Title: Efficacy outcomes of CDK4/6 inhibitors in combination with endocrine therapy treatment in hormone receptor-positive/HER2-negative advanced breast cancer according to PAM50 intrinsic subtype: Primary results of SOLTI-1801 CDK-PREDICT study
Authors : Pablo Tolosa , Tomás Pascual , Olga Martínez-Saez , Cristina Hernando , Sonia Servitja , María Fernández Abad , Fara Brasó-Maristany , Ester Sanfeliu , Javier David Benitez Fuentes , Laura Lema , Yolanda Ruano , Isabel García-Fructuoso , Lucía Parrilla , Adela Rodríguez , Ana María Roncero , María Ángeles Cobos , Rodrigo Sánchez-Bayona, Manuel Alva, Ainhoa Madariaga, Guillermo Villacampa Eva Ciruelos
Published in EJC Cancer in 2024
Background
Breast cancer (BC) is a leading malignancy globally and the most common histological subtype is hormone receptor-positive (HR+)/HER2-negative (HER2-). The introduction of cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) combined with endocrine therapy (ET) has become a standard in managing advanced HR+/HER2- BC, significantly extending progression-free survival (PFS) and, in some cases, overall survival (OS). Nevertheless the prognostic value of PAM50 intrinsic subtypes (IS), cell cycle, and immune-related gene expression in HR+/HER2- BC treated with CDK4/6i and ET in a first-line metastatic setting remains uncertain. This study aims to fill this gap by evaluating these biomarkers in metastatic biopsies from affected patients.
Methods
This ambispective, multicenter observational cohort study was conducted across six Spanish hospitals. Patients included were diagnosed with HR+/HER2- advanced breast cancer and treated in the first-line metastatic setting with CDK4/6 inhibitors combined with endocrine therapy. Baseline biopsies of metastatic lesions were collected before initiating therapy to assess PAM50 intrinsic subtypes and expression of cell cycle and immune-related genes.
- Primary Objective: Evaluate differences in progression-free survival (PFS) between PAM50 intrinsic subtypes using Cox regression models.
- Secondary Objectives: Assess overall survival (OS), overall response rate (ORR), and the correlation between gene expression markers and survival outcomes.
Study Design
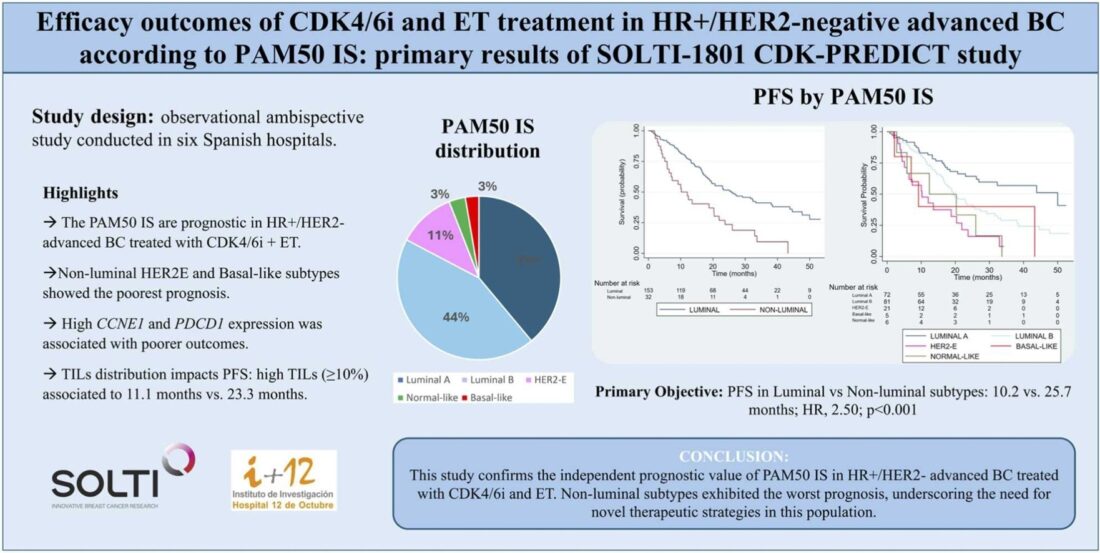
A total of 185 patients were enrolled in the study, with a median follow-up period of 38.5 months. Of the cohort, 82.7% had luminal subtypes (Luminal A and Luminal B), while 17.3% presented with non-luminal subtypes (HER2-enriched, Basal-like, and Normal-like).
Inclusion Criteria:
- Confirmed HR+/HER2- diagnosis based on ASCO/CAP guidelines.
- Biopsies collected within 90 days before starting CDK4/6i therapy.
- Eastern Cooperative Oncology Group (ECOG) performance status of 0-2.
Exclusion Criteria:
- Biopsies with inadequate tumor content.
- Early therapy discontinuation due to non-progression-related reasons.
Results
The study revealed significant differences in outcomes based on PAM50 intrinsic subtypes:
- Progression-Free Survival (PFS):
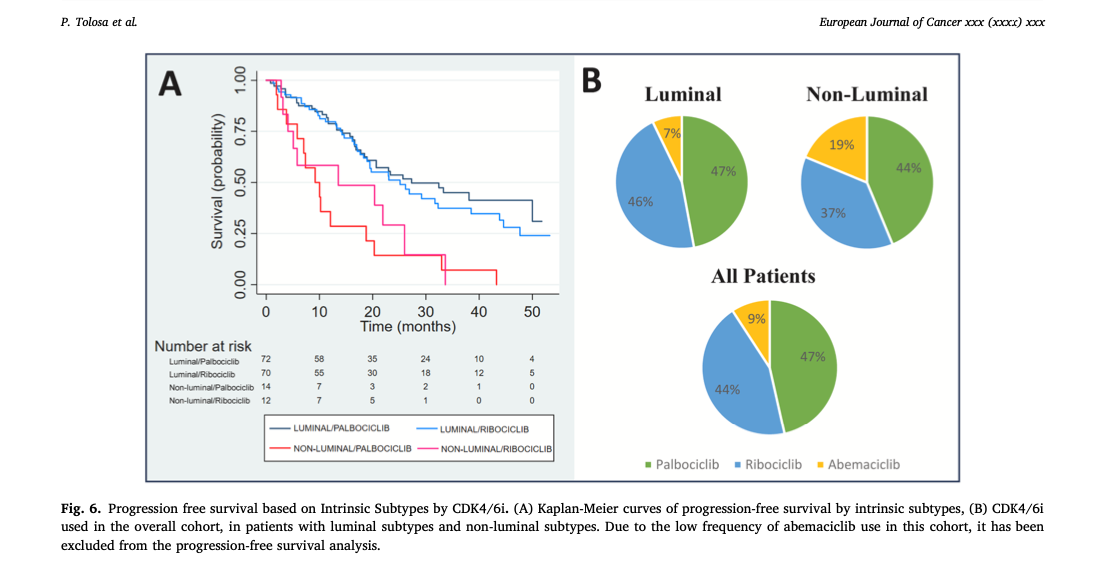
- Median PFS for luminal subtypes was 25.7 months compared to 10.2 months for non-luminal subtypes (Hazard Ratio [HR]: 2.50; p < 0.001).
- Among luminal subtypes, Luminal A had the best PFS at 50.0 months, while Luminal B had 19.5 months.
- Overall Survival (OS):
- Luminal subtypes demonstrated a median OS of 58.1 months, significantly longer than the 32.3 months observed for non-luminal subtypes (HR: 2.54; p < 0.001).
- Subtype-specific OS was highest for Luminal A (82.0 months) and lowest for Normal-like (20.0 months).
- Biomarker Analysis:
- Elevated expression of cell cycle genes, particularly CCNE1, and immune-related genes like PDCD1, correlated with poorer PFS and OS.
- Tumor-infiltrating lymphocytes (TILs) were associated with a worse prognosis, with patients exhibiting high TILs experiencing a median PFS of 11.1 months compared to 23.3 months in those with low TILs.
Key Findings
- Subtype-Driven Disparities:
- Non-luminal subtypes, including HER2-enriched and Basal-like, derived limited benefit from CDK4/6i and ET compared to luminal subtypes.
- Predictive Biomarkers:
- High CCNE1 expression suggests resistance to CDK4/6 inhibition, likely due to cell cycle dysregulation.
- Immune-related markers, particularly PDCD1, highlight the role of an immunosuppressive tumor microenvironment in therapy resistance.
- Therapeutic Implications:
- Non-luminal subtypes require alternative therapeutic strategies beyond current CDK4/6i and ET combinations, potentially including immune checkpoint inhibitors or novel targeted agents.
Key Takeaway Messages
- PAM50 subtypes offer critical prognostic insights: Luminal A patients benefit most from CDK4/6 inhibitors, while non-luminal subtypes face poorer outcomes.
- Biomarker analysis guides future strategies: Identifying high-risk patients through biomarkers like CCNE1 and PDCD1 enables more personalized treatment approaches.
- Targeted innovation is essential: Non-luminal subtypes exhibit inherent resistance to standard regimens, necessitating the exploration of novel therapies such as immune-modulating agents.
Conclusion
The SOLTI-1801 CDK-PREDICT study underscores the prognostic importance of PAM50 intrinsic subtypes in HR+/HER2- advanced breast cancer treated with CDK4/6 inhibitors and endocrine therapy. While luminal subtypes demonstrate favorable outcomes, non-luminal subtypes highlight an unmet clinical need for innovative therapeutic approaches. Biomarkers such as CCNE1, PDCD1, and TILs provide valuable tools for stratifying patients and tailoring treatment plans. These findings pave the way for further research into personalized oncology solutions that address the heterogeneity of advanced breast cancer.
Summary By Sona Karamyan, MD