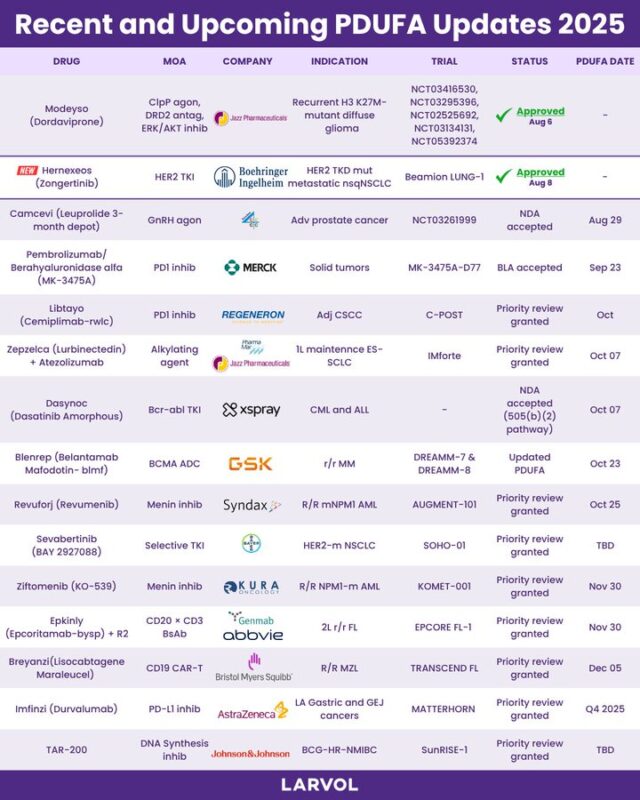
On August 8, 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval to zongertinib (Hernexeos, Boehringer Ingelheim Pharmaceuticals, Inc.), a kinase inhibitor, for adult patients with unresectable or metastatic non-squamous non-small cell lung cancer (NSCLC) harboring HER2 (ERBB2) tyrosine kinase domain (TKD) activating mutations—identified using an FDA-approved test—who have previously received systemic therapy.
Several Oncology Professionals Have Featured FDA’s accelerated approval to zongertinib on Social Media:
Marty Makary, 27th Commissioner of the U.S. FDA:
“The new FDA PreCheck program will help bring drug manufacturing to U.S. soil:
- Assist in plant design
- Inspect during construction
- Pre-approve to be FDA compliant immediately after construction
- Increase national security
- Advance President Trump’s America First Agenda.”
FDA Oncology shared on X:
“FDA grants accelerated approval to a treatment for non-small cell lung cancer.”

Amol Akhade, Consultant Medical Oncologist at Suyog Cancer Clinics:
FDA Accelerated Approval
Zongertinib (Hernexeos) for adults with HER2 (ERBB2) TKD-mutant unresectable/metastatic non-sq NSCLC after prior therapy 🫁
ORR: 75% (no prior HER2-ADC) | 44% (post HER2-ADC)
Daily oral TKI | Companion Dx: Oncomine Dx Target Test
Watch for hepatotoxicity, LV dysfunction, ILD/pneumonitis
A new targeted option — even post-T-DXd
Estela Rodriguez, Associate Director of Community Outreach-Thoracic Oncology and Co-Lead Thoracic Site Disease Group, Associate Director of Community Outreach at Sylvester Comprehensive Cancer Center:
“On this day, patients with HER2+ lung cancer got access to the first oral targeted therapy for HER2+ disease. -zongertinib
- Beamion trial 1L: ORR was 75% with 58% having a DOR ≥ 6 mos.
- previously treated with HER2-targeted ADC, ORR was 44% with 27% having a DOR ≥ 6 months.”

Vivek Subbiah, Chief of Early-Phase Drug Development at Sarah Cannon Research Institute:
“It’s a Friday and it’s time for another Precision Oncology approval FDA grants accelerated approval to zongertinib for non-squamous NSCLC with HER2 TKD activating mutations.
Stephen V Liu, Chief of Division of Hematology and Oncology at Georgetown Lombardi Comprehensive Cancer Center, Director of Thoracic Oncology and Developmental Therapeutics:
“FDA approves zongertinib 120mg po daily for previously treated advanced HER2 NSCLC. In the phase 1b Beamion-LUNG 1 study NEJM, RR 71% (42% post ADC), DOR 14.1m, intracranial RR 41%. Main toxicity is diarrhea (56%, 48% grade 1), no ILD.”
Fawzi Abu Rous, Thoracic Medical Oncologist at the Henry Ford Health:
“FDA grants accelerated approval to zongertinib for previously treated patients with HER2-mutated NSCLC, based on Phase 1b BEAMION-LUNG 1:
- RR 71% (42% post-ADC)
- DOR 14.1 mo
- Intracranial RR 41%
Main AE: diarrhea, no ILD
Great news for patients — this is the first oral option for this population.”
Balazs Halmos, Associate Director for Clinical Science at Montefiore Einstein Cancer Center:
“First impressions.”

Devika Das, Physician Director of Quality at SHAND’S HOSPITAL OF UF:
“This is an important approval in lcsm
HER2 mutations are often under recognized as a target and now that there is an oral agent with *fairly tolerable* safety profile, it’s prudent that we test not only prospectively but for those patients who might have missed getting NGS front line. ( or go back and review the NGS reports again!).”
LARVOL shared on X:
“On August 8, 2025, the FDA grants accelerated approval to zongertinib for non-squamous NSCLC with HER2 TKD activating mutations. Learn more about Beamion LUNG-1 (NCT04886804).”