Mismatch repair–deficient (dMMR)/microsatellite instability–high (MSI-H) cancers are highly immunogenic and respond to immune checkpoint inhibitors. While anti–PD-1 monotherapy has shown durable benefit in about one-third of patients with advanced dMMR/MSI-H noncolorectal cancers, combined PD-1/CTLA-4 blockade may enhance efficacy. The MOST-CIRCUIT trial is the first study to evaluate nivolumab plus ipilimumab in this population.
You Can Read about Opdivo (Nivolumab): Uses in Cancer, Side Effects, Dosages, Expectations, and More
Study Design and Methods
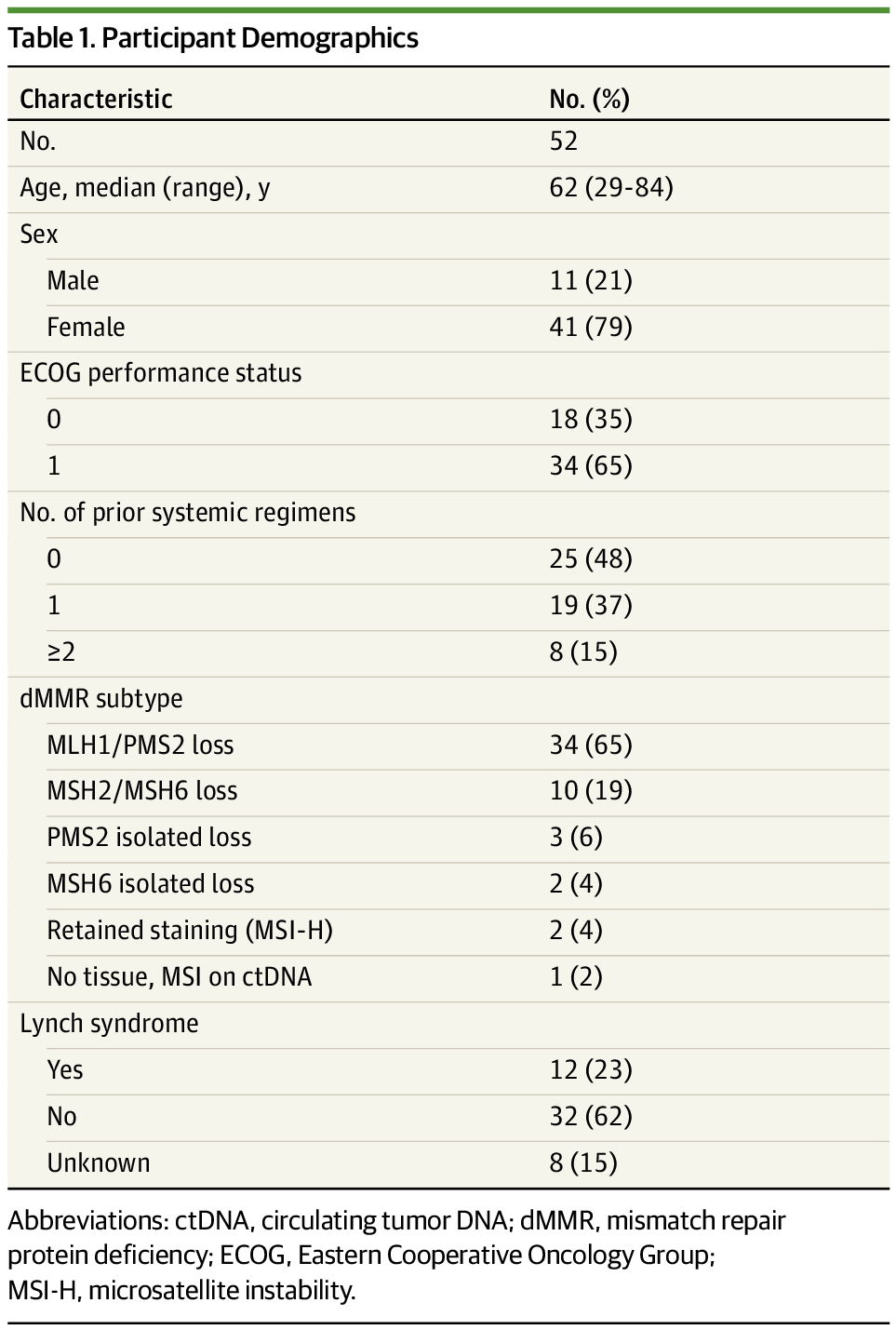
This was a phase 2, multicenter, nonrandomized clinical trial conducted across Australia and New Zealand. A total of 52 patients with advanced dMMR/MSI-H noncolorectal cancers were enrolled, representing 17 different tumor types, with endometrial cancer being the most common (50% of the cohort).
Patients received a combination immunotherapy regimen beginning with nivolumab (3 mg/kg) and ipilimumab (1 mg/kg) administered every three weeks for four cycles. After this induction phase, patients continued on nivolumab 480 mg every four weeks for up to 96 weeks, or until disease progression or unacceptable toxicity.
The trial evaluated two co-primary endpoints:
- Objective Response Rate (ORR) according to RECIST 1.1
- 6-month progression-free survival (PFS)
Secondary endpoints included overall survival (OS), median PFS, and the safety profile of the combination regimen.
Results
- ORR: 63% (95% CI, 50–75%)
- 6-month PFS: 71% (95% CI, 57–81%)
- Median PFS and OS: Not reached
- Duration of Response: Not reached; 79% ongoing responses
- Grade 3/4 immune-related AEs: 23% (12 patients)
- Most common tumor: Endometrial (26/52 patients, 50%)
- 52% were pretreated for metastatic disease
Key Findings
- Combined nivolumab + ipilimumab demonstrated high and durable response rates across multiple dMMR/MSI-H noncolorectal cancers.
- Activity compares favorably to historical outcomes with PD-1/PD-L1 monotherapy.
- Manageable safety profile consistent with known immune-related toxicities.
You Can Read All Article Here