Ivermectin, best known as an antiparasitic drug that transformed global health, is now being investigated for a surprising new role in oncology. Laboratory and preclinical studies suggest that ivermectin may interfere with cancer cell growth, promote tumor cell death, and even enhance immune recognition of tumors.
These intriguing findings have led researchers to explore whether the drug can be safely and effectively combined with immunotherapy in aggressive cancers where treatment options remain limited.
What Is Ivermectin?
Ivermectin is a well-known antiparasitic drug, originally developed in the late 1970s and widely used since the 1980s to treat parasitic infections such as river blindness (onchocerciasis), lymphatic filariasis, and strongyloidiasis. It works by binding to glutamate-gated chloride channels in parasites, leading to paralysis and death of the organism. Its discovery and global distribution revolutionized parasitic disease control, and the scientists behind it were awarded the 2015 Nobel Prize in Medicine.
Because ivermectin has an established safety profile, is inexpensive, and is widely available, researchers have explored whether it can be “repurposed” for other diseases beyond parasitic infections—including cancer.
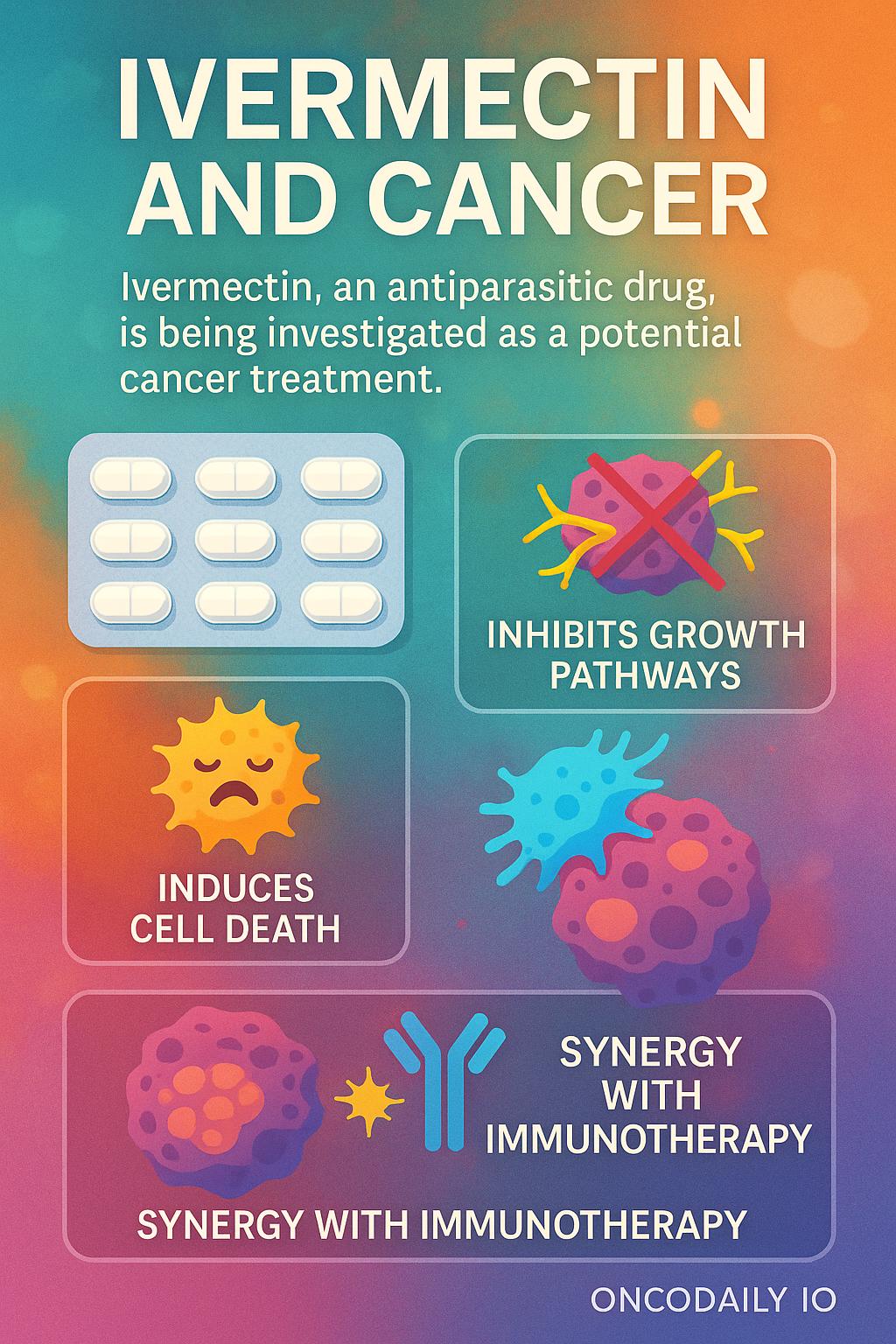
Why Consider Ivermectin in Cancer?
Several laboratory and preclinical studies suggest that ivermectin has properties that may interfere with cancer cell survival. Proposed mechanisms include:
- Inhibition of tumor growth pathways: Ivermectin appears to block PAK1 and WNT-TCF signaling, which are involved in cancer proliferation and metastasis.
- Induction of programmed cell death (apoptosis): It can trigger mitochondrial dysfunction, leading to cancer cell death.
- Impact on tumor microenvironment: Some evidence shows ivermectin may reduce cancer stem-like cells and improve immune recognition of tumors.
- Synergy with immunotherapy: Preclinical models suggest that ivermectin can enhance the activity of immune checkpoint inhibitors, potentially overcoming resistance in hard-to-treat cancers.
These findings, while preliminary, led to interest in testing ivermectin clinically in oncology.
Exploring Ivermectin in Cancer Treatment
Outside of oncology, ivermectin has been a subject of public debate, particularly during the COVID-19 pandemic, where it was promoted by some as a potential antiviral despite limited evidence. While this fueled controversy, it also highlighted a broader public interest in “repurposing” old drugs for new diseases. In cancer, the scientific rationale is stronger, but robust clinical evidence is still lacking.
Ivermectin Plus Immunotherapy in Metastatic Triple-Negative Breast Cancer
Triple-negative breast cancer (TNBC) remains one of the most aggressive breast cancer subtypes, often associated with early relapse and limited treatment options once metastatic. Despite advances with immune checkpoint inhibitors such as pembrolizumab, outcomes remain poor for patients who progress after frontline therapy. Novel drug combinations are urgently needed to improve both survival and quality of life in this population.
This ongoing phase I/II study (NCT identifier: IIT2022-07-YUAN-IB-TNBC) is evaluating the safety, tolerability, and efficacy of combining ivermectin, an antiparasitic drug with potential anticancer properties, with balstilimab or pembrolizumab in patients with metastatic TNBC.
Study Rationale
Checkpoint inhibitors enhance immune activation against tumors, but many patients with TNBC do not achieve durable responses. Preclinical data suggest that ivermectin may interfere with tumor growth by blocking pathways that support cancer cell survival and immune evasion. Combining ivermectin with PD-1 antibodies could potentially enhance tumor shrinkage and long-term control.
Study Design
- Type: Phase I/II, single-arm, interventional, open-label
- Participants: 34 adults with histologically confirmed metastatic TNBC (Stage IV, AJCC v8), ECOG 0–1, who have progressed on 1–2 prior systemic regimens.
- Location: Cedars-Sinai Medical Center, Los Angeles, USA
- Start date: October 13, 2023
- Estimated completion: October 2026
Treatment protocol
- Ivermectin orally on Days 1–3, 8–10, 15–17 of each 21-day cycle.
- Balstilimab (450 mg IV) or Pembrolizumab (200 mg IV) on Day 1 of each cycle.
- Treatment continues for up to 35 cycles (approximately 2 years) or until progression, toxicity, or withdrawal.
- Patients are followed for 90 days post-treatment and then periodically thereafter.
Eligibility Criteria
To participate in this trial, patients must be adults aged 18 years or older with an ECOG performance status of 0–1 and a life expectancy of more than three months. They should have histologically confirmed metastatic triple-negative breast cancer (TNBC), defined by estrogen receptor (ER) and progesterone receptor (PR) expression of 10% or less and HER2 negativity by either immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH). Eligible participants must also have experienced disease progression after one or two prior systemic therapies in the metastatic setting and demonstrate adequate organ function and preserved cardiac function.
Patients will not be eligible if they have received prior PD-1/PD-L1 inhibitor therapy in the metastatic setting. Other exclusion factors include the presence of active autoimmune disease, uncontrolled infections, or active central nervous system (CNS) metastases or carcinomatous meningitis. Individuals with a history of pneumonitis requiring steroid treatment are also excluded, as are those who have received a live vaccine within 30 days prior to starting study treatment.
You Can Also Read About Triple-Negative Breast Cancer: Symptoms ,Causes, Types, Diagnosis and Treatment
Written by Toma Oganezova, MD, Editor-in-Chief of OncoDaily IO


