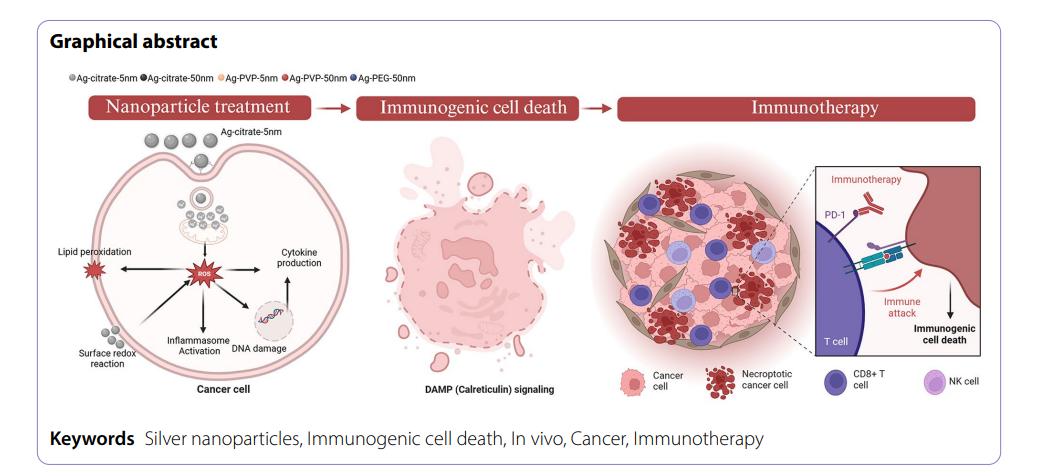
This study demonstrates the potential of silver nanoparticles (Ag-citrate-5 nm) as an immunomodulatory agent to enhance immune checkpoint blockade (ICB) therapy, particularly anti-PD1, in the treatment of tumors with immunosuppressive tumor microenvironments (TME). The combination of Ag-citrate-5 nm and anti-PD1 significantly boosted tumor suppression by inducing immunogenic cell death (ICD), which enhanced immune cell infiltration, particularly CD8+ T cells, and tumor-associated macrophages (TAMs). The role of CD8+ T cells was confirmed, as their depletion reduced the efficacy of the treatment, indicating their essential role in tumor control. Ag-citrate-5 nm’s ability to induce ICD and activate systemic immune responses suggests its potential for long-term immunological memory, offering a safe and effective strategy for improving immunotherapy outcomes without significant toxicity to vital organs.
Authors
Ara Sargsian , Xanthippi Koutsoumpou, Hermon Girmatsion, Can Egil , Kiana Buttiens, Carla Rios Luci , Stefaan J Soenen ,Bella B Manshian
Published in Journal of Nanobiotechnology, Oct 2024
Background
The efficacy of immune checkpoint blockade (ICB) in certain tumor types is often limited due to immunosuppressive tumor microenvironments (TME) that lack tumor-infiltrating immune cells. Silver nanoparticles (Ag NPs) have emerged as potential immunomodulatory agents that may enhance immune responses. This study investigates the therapeutic potential of Ag-citrate-5 nm in combination with anti-PD1 ICB in Renca tumor models.
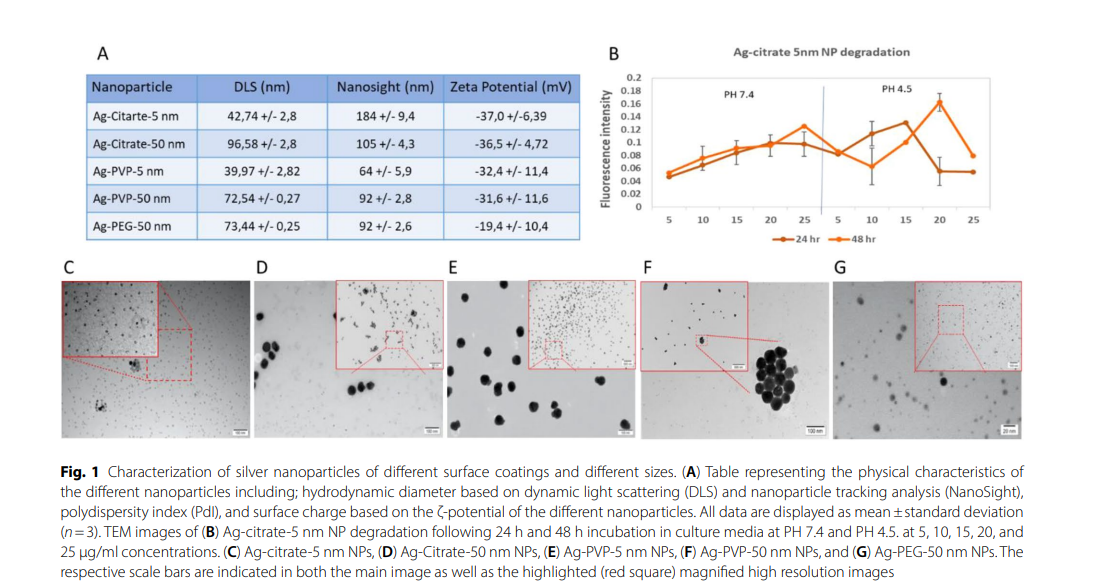
Methods
A series of in vivo experiments were conducted using Renca-Luc+ tumor-bearing mice. Treatment groups included peritumoral injection of Ag-citrate-5 nm (20 µg/mouse), intraperitoneal injection of anti-PD1 antibody (150 µg/mouse), and a combination of both. Control groups included PBS treatment and additional groups with anti-CD8 antibodies to assess the role of cytotoxic T cells. Tumor progression was monitored using bioluminescence imaging, and immune responses were evaluated through flow cytometry, histological analysis, and cytokine profiling.
Study Design
The study examined the effect of Ag-citrate-5 nm and anti-PD1 both as monotherapies and in combination. Tumor-bearing mice were treated every four days with three doses of anti-PD1, either alone or in combination with Ag-citrate-5 nm. Additional control groups received anti-CD8 antibodies to deplete CD8+ T cells, assessing their contribution to therapeutic efficacy. Bioluminescence imaging and immune cell infiltration analysis were conducted to evaluate tumor response and immune activation. Evaluation markers were Tumor growth (bioluminescence imaging), immune cell infiltration (flow cytometry, fluorescence imaging), systemic immune activation (CD4+ and CD8+ T cells in spleen), nflammatory markers (caspase, elastase activity).
Treatment Groups:
- Control (PBS)
- Ag-citrate-5 nm alone
- Anti-PD1 alone
- Combination of Ag-citrate-5 nm with anti-PD1
- Additional groups receiving anti-CD8 to deplete cytotoxic T cells
Results of Silver nanoparticle induced immunogenic cell death and immunotherapy
Tumor Response and Immune Activation
While neither Ag-citrate-5 nm nor anti-PD1 monotherapy significantly reduced tumor burden, their combination demonstrated a substantial therapeutic effect. Tumor flux was significantly lower in the combination group compared to control and monotherapy groups, indicating a synergistic effect. Increasing Ag-citrate-5 nm (50 µg/mouse) and anti-PD1 (200 µg/mouse) concentrations further enhanced tumor suppression.
Role of CD8+ T Cells
CD8+ T cell depletion largely abrogated the therapeutic efficacy of the Ag-citrate-5 nm and anti-PD1 combination, confirming the importance of cytotoxic T cells in tumor control. However, residual tumor suppression despite CD8+ depletion suggests additional immune mechanisms at play, potentially involving tumor-associated macrophages (TAMs), which are known to express PD1.
Immune Cell Infiltration and Activation
Histological analysis revealed increased infiltration of CD8+ T cells and macrophages in tumor tissues treated with Ag-citrate-5 nm and anti-PD1. Flow cytometry analysis of splenocytes showed elevated CD4+ and CD8+ T cell levels in the combination group. Additionally, significant increases in CD69+ and CD38+ T cells suggested heightened systemic immune activation.
Induction of Immunogenic Cell Death (ICD)
Ag-citrate-5 nm treatment induced calreticulin translocation to the tumor cell surface, a hallmark of ICD. Despite the presence of neutrophil elastase, which can promote tumor progression, the combination treatment did not increase caspase activation, supporting ICD rather than apoptotic cell death as the predominant mechanism.
Key Findings
This study provides compelling evidence that Ag-citrate-5 nm can serve as a potent immunomodulatory agent when combined with anti-PD1 ICB. By inducing ICD and enhancing immune cell infiltration, Ag-citrate-5 nm overcomes the limitations of ICB in immunosuppressive TMEs.
- Ag-citrate-5 nm enhances anti-PD1 efficacy, leading to significant tumor suppression.
- CD8+ T cells play a crucial role in mediating the anti-tumor effects of the combination therapy.
- Ag-citrate-5 nm induces ICD, attracting immune cells to the tumor microenvironment.
- Systemic immune activation, marked by increased CD69+ and CD38+ T cells, suggests potential for long-term immunological memory.
- No significant toxicity was observed in vital organs, supporting the safety of Ag-citrate-5 nm.
Key Takeaway Messages
- Silver Nanoparticles Enhance Immunotherapy: Ag-citrate-5 nm significantly boosts the efficacy of anti-PD1 treatment by inducing ICD and promoting immune cell infiltration.
- CD8+ T Cells Are Essential: Depleting CD8+ T cells reduced treatment efficacy, emphasizing their importance in tumor control.
- Systemic Anti-Tumor Immunity: Increased activation of CD69+ and CD38+ T cells in the spleen indicates the potential for lasting immune protection.
- Safe and Effective Combination: No structural damage was observed in liver, lung, or kidney tissues, supporting the biocompatibility of Ag-citrate-5 nm.
Future research should explore different dosing strategies, administration routes, and validation in orthotopic tumor models to optimize clinical translation.
You can read the full article here


