At the ESMO Congress 2025, Dr. Andrea Necchi (Milan, Italy) presented results from the GDFather-NEO trial (NCT06059547), evaluating the novel GDF-15 inhibitor visugromab (20 mg/kg) in combination with nivolumab (480 mg) compared to nivolumab plus placebo in patients with muscle-invasive bladder cancer (MIBC) who were cisplatin-ineligible or declined chemotherapy.
Methods
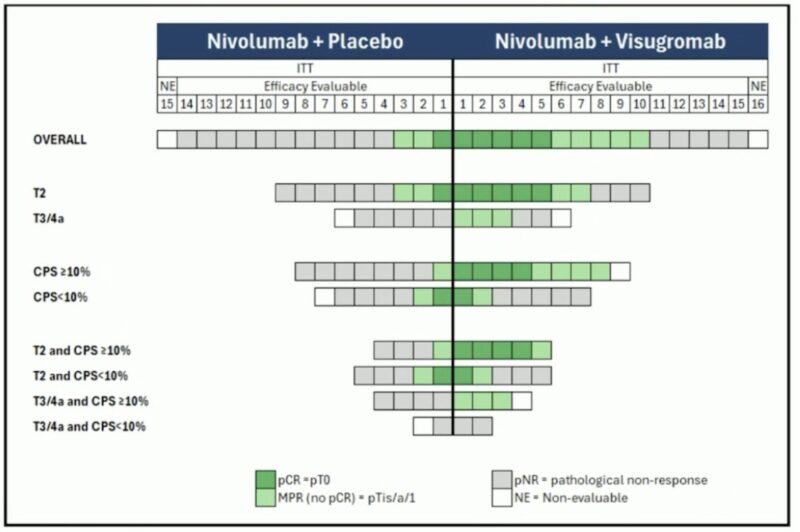
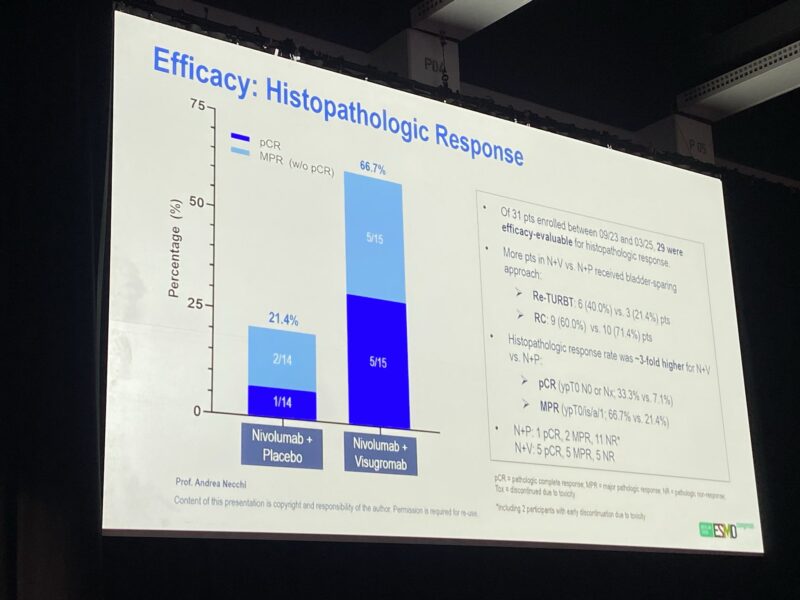
This multicenter phase II study enrolled 31 patients, with 28 evaluable for efficacy. The visugromab plus nivolumab (N/V) arm achieved markedly higher efficacy compared to nivolumab plus placebo (N/P), including:
- Pathologic complete response (pCR): 33.3% vs. 7.7%
- Major pathologic response (MPR): 66.7% vs. 23.1%
- Objective response rate (ORR, RECIST v1.1): 60% (7 CR, 2 PR) vs. 15.4% (0 CR, 2 PR)
Results
Among the 31 patients enrolled (September 2023–March 2025), 28 were included in the efficacy analysis: 15 received nivolumab + visugromab (N/V) and 13 received nivolumab + placebo (N/P). After therapy, 6 patients in the N/V group and 3 in the N/P group underwent a repeat TURBT instead of cystectomy.
Efficacy outcomes strongly favored the N/V combination:
- Pathologic complete response (pCR):
33.3% with N/V vs 7.7% with N/P - Major pathologic response (MPR):
66.7% with N/V vs 23.1% with N/P - Radiologic objective response rate (ORR):
60% (7 complete responses, 2 partial responses) with N/V
vs 15.4% (2 partial responses, no complete responses) with N/P
The benefit of the N/V combination was seen across all tumor stages, with especially strong responses in patients with PD-L1 CPS ≥10%.
Safety
N/V was well tolerated, with no unexpected side effects reported; detailed safety results will be presented. Baseline GDF-15 levels were similar between groups, confirming that the difference in treatment response was not due to baseline GDF-15 differences.
Conclusion
These findings demonstrate that visugromab plus nivolumab tripled the response rate compared with nivolumab alone, positioning GDF-15 blockade as a promising new strategy in neoadjuvant MIBC therapy. Further trials are warranted to confirm efficacy and explore tumor response–guided bladder preservation approaches.

You Can Also Read INTEGRATE IIb Trial at ESMO 2025: Regorafenib Plus Nivolumab vs Chemotherapy in Advanced Gastric and GEJ Cancer by OncoDaily
You Can Also Read Full Abstract Here


