At the ESMO Congress 2025 in Berlin, Professor David Goldstein (NSW, Australia) presented the results of the Phase III INTEGRATE IIb trial (LBA80, NCT04879368) — a global, randomized study led by the Australasian Gastrointestinal Trials Group (AGITG) in collaboration with international partners.
This pivotal trial evaluated regorafenib plus nivolumab (RegoNivo) versus investigator’s choice of chemotherapy in patients with previously treated advanced gastric or gastroesophageal junction (GEJ) cancer. While the study did not meet its primary endpoint of overall survival (OS) superiority, RegoNivo demonstrated higher response rates, longer duration of benefit, and improved quality-of-life outcomes — underscoring the ongoing need to refine non-chemotherapy combinations for refractory disease.
Background
Patients with refractory advanced gastric and GEJ cancers face limited treatment options after progression on platinum- and fluoropyrimidine-based therapies. The INTEGRATE IIb trial followed earlier phase data suggesting that combining regorafenib, a multi-kinase inhibitor with antiangiogenic and immunomodulatory properties, with nivolumab, a PD-1 inhibitor, might enhance anti-tumor activity and immune responsiveness.
This study aimed to determine whether RegoNivo could improve survival outcomes compared with standard chemotherapy options such as taxanes, irinotecan, or trifluridine/tipiracil in this difficult-to-treat population.
Methods
Eligible patients who had received two or more prior lines of systemic therapy were randomized 2:1 to receive either:
- Regorafenib (90 mg orally, days 1–21, every 28 days) plus
Nivolumab (240 mg IV, every 2 weeks),
or - Investigator’s choice chemotherapy (taxane, irinotecan, or trifluridine/tipiracil).
The primary endpoint was overall survival (OS). Secondary endpoints included progression-free survival (PFS), objective response rate (ORR), quality of life (QoL), and safety.

Results
Among 462 patients enrolled globally, 309 received RegoNivo and 153 received chemotherapy.
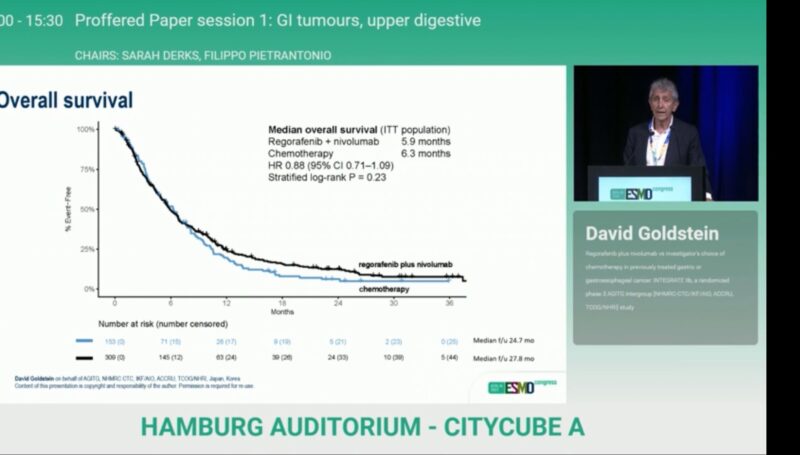
Median OS was 5.9 months for RegoNivo and 6.3 months for chemotherapy (HR 0.88; 95% CI 0.71–1.09; P = 0.23).
Progression-free survival was comparable between groups (1.9 vs 1.9 months).
However, objective response rate (ORR) favored RegoNivo (7.4% vs 2.6%), with a median duration of response of 9.5 vs 5.6 months.
The disease control rate (DCR) was also higher with RegoNivo (39% vs 26%) and maintained at 12 months (14% vs 0%).
Importantly, global quality-of-life deterioration or death was delayed with RegoNivo (HR 0.74; 95% CI 0.60–0.91), highlighting a patient-centered benefit despite no survival advantage.
Serious adverse events occurred in both arms but were consistent with known safety profiles. Most treatment-related adverse events with RegoNivo were manageable, with no new safety signals identified.
Conclusions
The INTEGRATE IIb trial showed that regorafenib plus nivolumab did not significantly improve overall survival compared with standard chemotherapy in refractory gastric and GEJ cancers.
Nonetheless, the higher response rate, longer duration of benefit, and better quality-of-life outcomes indicate biological activity and justify continued investigation into next-generation regorafenib-based combinations and non-chemotherapy regimens in gastric cancer.
These findings reinforce the complexity of overcoming resistance in late-line settings while emphasizing the promise of immunotherapy-based combinations for future clinical exploration.
You Can Also Read MATTERHORN Trial at ESMO 2025: Durvalumab Plus FLOT in Resectable Gastric and GEJ Adenocarcinoma by OncoDaily

You Can Also Read Full Abstract Here


