At the ESMO Congress 2025 in Berlin, Professor Josep Tabernero (Vall d’Hebron Institute of Oncology, Barcelona, Spain) presented the final overall survival (OS) results from the Phase III MATTERHORN trial (NCT04592913) — a global, double-blind study.
This pivotal study evaluated Durvalumab (Imfinzi) plus FLOT chemotherapy (5-fluorouracil, leucovorin, oxaliplatin, and docetaxel) versus placebo plus FLOT in patients with resectable gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. The final analysis, presented in the Proffered Paper Session (LBA81), now confirms a statistically significant overall survival advantage, supporting durvalumab plus FLOT as a potential new standard in the perioperative setting.
At OncoDaily GI, we spotlight the breakthroughs shaping gastrointestinal oncology — from immunotherapy in early-stage disease to global trials redefining curative strategies.
Background
Gastric and gastroesophageal junction cancers remain among the leading causes of cancer mortality worldwide, with many patients diagnosed at a stage requiring multimodal therapy. For those with resectable disease, perioperative FLOT chemotherapy is the current standard of care and has improved long-term outcomes compared with older regimens.
However, recurrence rates remain substantial, with more than half of patients eventually relapsing after curative surgery.
In metastatic disease, immune checkpoint inhibitors targeting PD-1 or PD-L1 have transformed treatment paradigms, delivering durable responses and survival benefits. These successes have fueled efforts to integrate immunotherapy earlier in the treatment course, particularly in the perioperative setting, where the goal is to reduce recurrence and improve cure rates.
The MATTERHORN trial was designed to test whether combining durvalumab, a PD-L1 inhibitor, with FLOT could further enhance anti-tumor activity and long-term survival outcomes compared with chemotherapy alone in patients with resectable G/GEJ adenocarcinoma.Methods
MATTERHORN Trial Design
MATTERHORN was a global, double-blind, randomized Phase III trial enrolling patients with resectable gastric or gastroesophageal junction adenocarcinoma.
Participants were randomized 1:1 to receive either:
- Durvalumab 1500 mg every 4 weeks or placebo,
- in combination with FLOT chemotherapy every 2 weeks for four cycles — two preoperative and two postoperative —
- followed by ten additional cycles of durvalumab or placebo monotherapy.
The primary endpoint was event-free survival (EFS), and overall survival (OS) was a key secondary endpoint.
The trial also assessed pathological complete response (pCR), major pathological response (MPR), and the association of pathological outcomes and nodal status (ypN) with survival.

Results of MATTERHORN
Earlier this year, in June 2025, the interim efficacy results of trial were published in the NEJM by Yelena Y. Janjigian, Salah-Eddin Al-Batran, Zev A. Wainberg, Kei Muro, Eric Van Cutsem, Woo Jin Hyung, Josep Tabernero, and colleagues. This global, randomized, double-blind study compared perioperative durvalumab plus FLOT (D + FLOT) with placebo plus FLOT in 948 patients with resectable, locally advanced gastric or gastroesophageal junction cancer.
At a median follow-up of 31.5 months, D + FLOT significantly improved event-free survival (HR 0.71; 95% CI 0.58–0.86; p<0.001), with median EFS not reached versus 32.8 months for placebo. Two-year EFS rates were higher with D + FLOT (67.4% vs 58.5%), and overall survival trended favorably (HR 0.78) with final OS data awaited. Durvalumab did not delay surgery or adjuvant therapy, and grade 3–4 adverse events were comparable between arms. These results support D + FLOT as a potential new standard of care for resectable gastric and GEJ cancer.
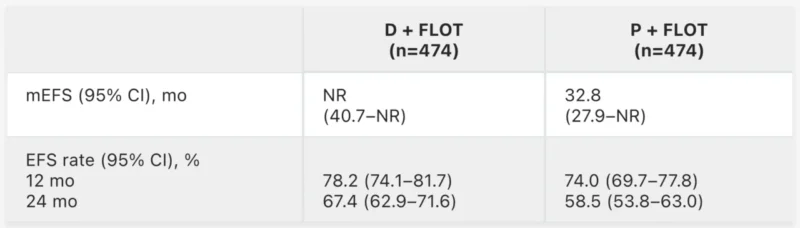
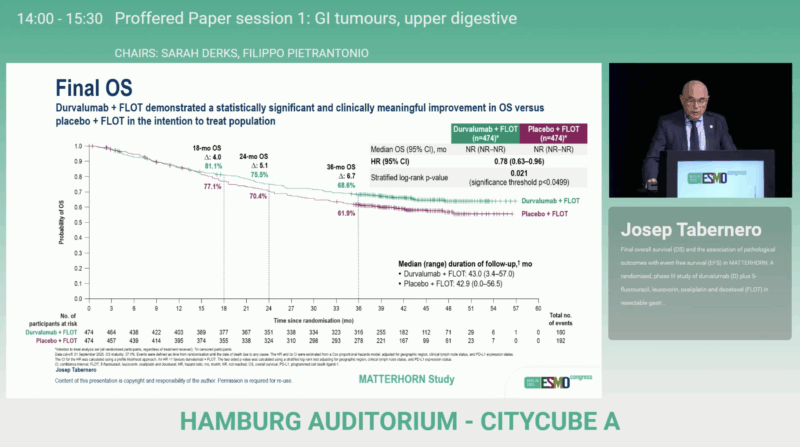
At the ESMO Congress 2025, Prof. Tabernero presented the final overall survival analysis, which showed a statistically significant and clinically meaningful OS improvement for durvalumab + FLOT compared with placebo + FLOT (HR 0.78; 95% CI 0.63–0.96; p = 0.021).
The OS benefit was consistent regardless of PD-L1 expression, with similar hazard ratios across TAP < 1% and TAP ≥ 1% subgroups.
Additionally, patients receiving durvalumab achieved higher nodal negativity rates (ypN– 58.2% vs 44.8%), reflecting greater tumor regression.
Event-free survival improvements were observed across all levels of pathological response — including partial and major responders — underscoring that the immunotherapy benefit extended beyond those achieving complete response.
Conclusions
The MATTERHORN trial demonstrated that perioperative durvalumab combined with FLOT chemotherapy significantly improves both overall and event-free survival in patients with resectable gastric and gastroesophageal junction adenocarcinoma.
The treatment effect was independent of PD-L1 status or nodal involvement, reinforcing the robustness of the benefit across subgroups.
Together with the previously published JCO data, these findings position durvalumab + FLOT as a potential new global standard of care for patients undergoing curative-intent treatment for gastric and GEJ adenocarcinoma and further highlight the expanding role of checkpoint inhibition in early-stage gastrointestinal cancers.
You can read the full abstract here.