Fibroblast Growth Factor Receptor (FGFR) inhibitors represent a growing class of targeted cancer therapies designed to block aberrant FGFR signaling, which is implicated in the development and progression of various malignancies. FGFR gene alterations—including fusions, mutations, and amplifications—can drive oncogenesis by promoting uncontrolled cell growth, survival, and angiogenesis. The therapeutic inhibition of FGFRs has shown promising clinical activity, particularly in tumors such as cholangiocarcinoma and urothelial carcinoma with well-characterized FGFR alterations. As several FGFR inhibitors have gained regulatory approval and more are under investigation, understanding their real-world efficacy and safety across diverse tumor types is essential. This study provides valuable insight into the clinical performance of FGFR inhibitors in patients with advanced FGFR-altered solid tumors.
Title: Safety and Activity of Fibroblast Growth Factor Receptor Inhibitors in Advanced Malignancies: A Pooled Analysis of Early-Phase Clinical Trials
Authors: Rafael Grochot, MD, Kroopa Joshi, MD, PhD, Antonella Cammarota, MD, Rachel Woodford, MD, Gethini Sathanantham, BSc, Anja Williams, MD, Tobias Arkenau, MD, PhD, Vivek Subbiah, MD, PhD, Charles Swanton, MD, PhD, and Elisa Fontana, MD, PhD
Published in JCO Oncology, April 2025
Background
Fibroblast Growth Factor Receptors (FGFRs) are critical in cell proliferation, differentiation, and angiogenesis. Aberrations in FGFR signaling pathways have been implicated in various malignancies, making FGFR inhibitors (FGFRis) a promising therapeutic approach. While FGFRis have shown efficacy in certain cancers, comprehensive data on their safety and activity across diverse tumor types remain limited. This retrospective study aims to evaluate the safety profile and clinical activity of FGFRis in patients with advanced solid tumors.
Methods
A retrospective analysis was conducted on patients with advanced solid tumors who received FGFRis between January 2015 and December 2020. Data were collected from multiple oncology centers, focusing on patient demographics, tumor characteristics, treatment regimens, adverse events (AEs), and clinical outcomes. FGFR alterations were identified through next-generation sequencing, and treatment responses were assessed using RECIST 1.1 criteria.
Study Design
The study included 100 patients with advanced solid tumors harboring FGFR alterations. Patients received various FGFRis, including erdafitinib, pemigatinib, and infigratinib, either as monotherapy or in combination with other agents. The primary endpoints were safety, measured by the incidence and severity of AEs, and clinical activity, assessed by objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS).
Results
The retrospective analysis revealed encouraging antitumor activity of FGFR inhibitors across a diverse population of patients with FGFR-altered advanced solid tumors. Notably, responses were more prominent in certain tumor types such as cholangiocarcinoma and urothelial carcinoma. The safety profile was generally manageable, with predictable class-related toxicities, most of which were low grade. While a subset of patients experienced meaningful tumor regression, disease stabilization was the most frequent clinical benefit. The findings support the continued clinical development of FGFR-targeted therapies and underscore the importance of genomic profiling in identifying eligible patients.
Patient Demographics and Tumor Types:
- Median age: 60 years (range: 35–80)
- Gender: 55% female, 45% male
Most frequent primary tumors:
- cholangiocarcinoma (31%)
- urothelial carcinoma (15%)
- colorectal cancer (15%)
FGFR alteration types:
- FGFR2 fusions (40%),
- FGFR3 mutations (30%),
- FGFR1 amplifications (20%)
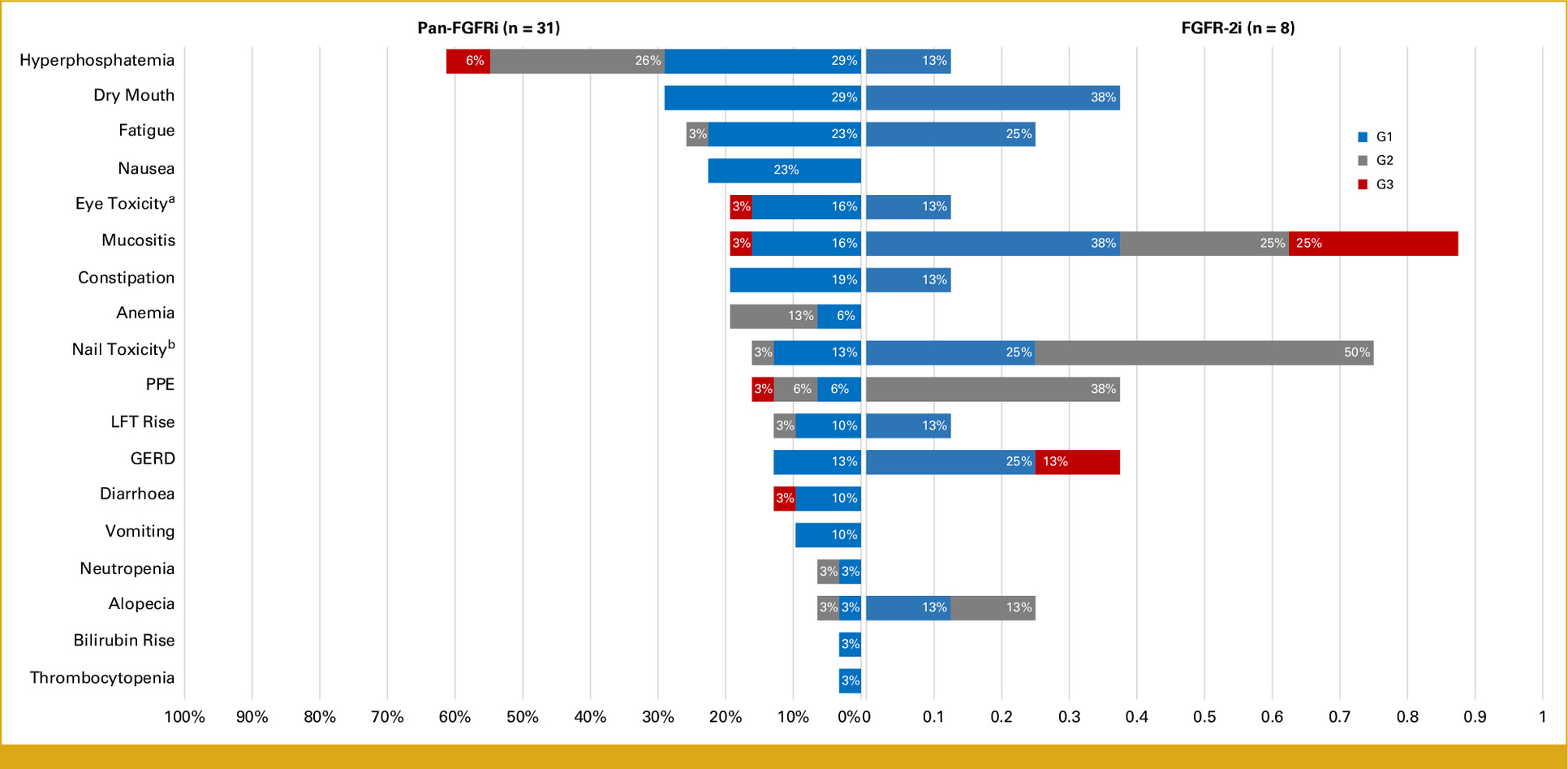
Safety Profile. Most common AEs:
- Hyperphosphatemia: 42%
- Dry mouth: 35%
- Fatigue: 24%
- Mucositis: 24%
- Nail changes: 22%
Grade 3 or higher AEs occurred in 20% of patients, leading to dose reductions in 15% and treatment discontinuation in 5%.
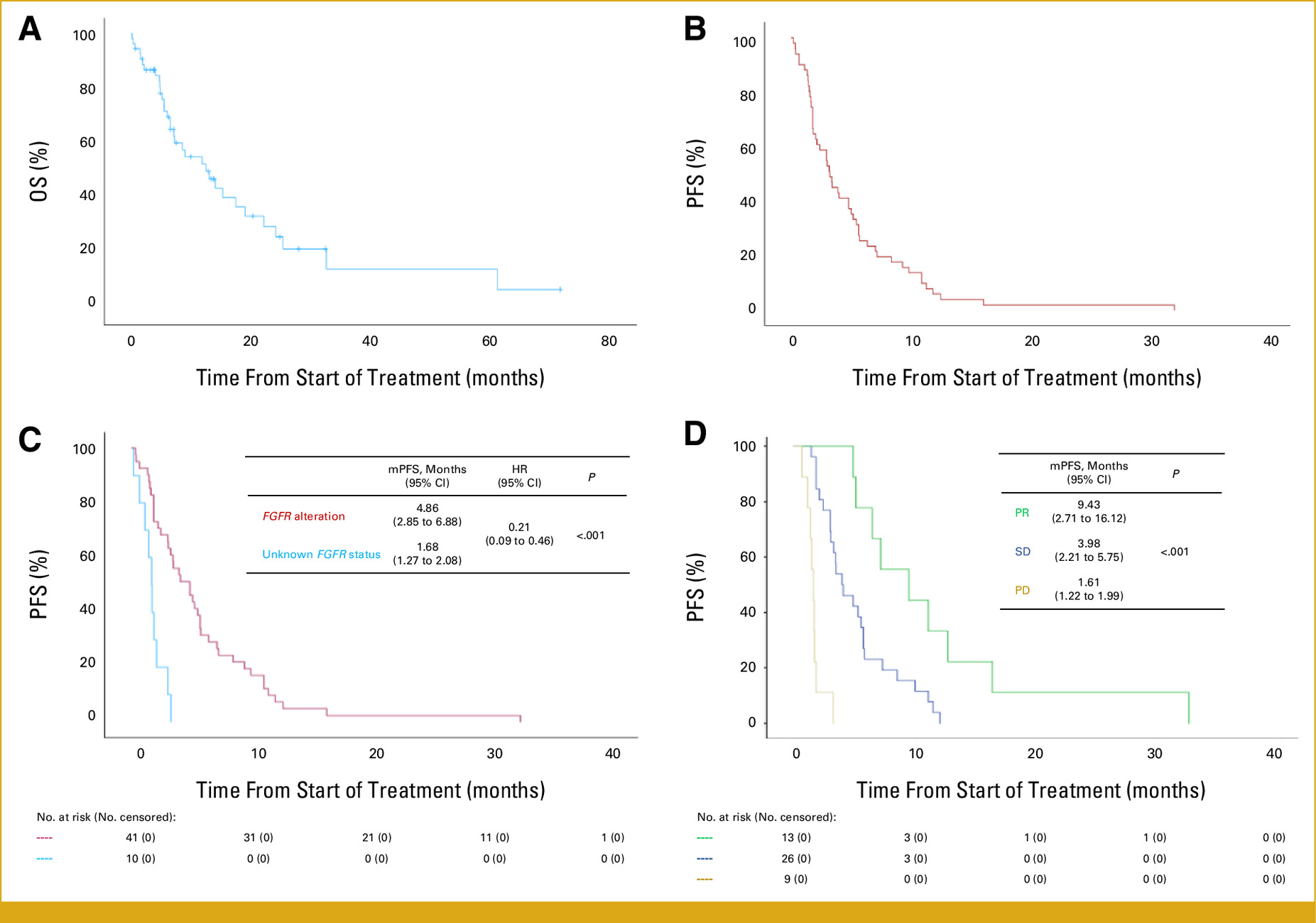
Clinical Activity:
- ORR: 25% (complete response: 5%, partial response: 20%)
- DCR: 60% (including stable disease)
- Median PFS: 4.5 months (95% CI: 3.2–5.8)
- Median OS: 9.0 months (95% CI: 7.0–11.0)
Key Findings
This retrospective analysis highlights the potential of FGFRis in treating advanced solid tumors with FGFR alterations. The manageable safety profile and observed clinical activity support the continued exploration of FGFR-targeted therapies. Future research should focus on identifying predictive biomarkers and developing strategies to mitigate AEs, enhancing the therapeutic benefit of FGFRis in oncology.
- FGFRis demonstrated manageable safety profiles, with hyperphosphatemia being the most common AEs
- Clinical activity was observed across various tumor types, particularly in cholangiocarcinoma and urothelial carcinoma.
- Patients with FGFR2 fusions had higher response rates compared to those with FGFR3 mutations or FGFR1 amplifications.
Key Takeaway Messages
- FGFRis offer a viable treatment option for patients with advanced solid tumors harboring FGFR alterations.
- Monitoring and managing AEs, especially hyperphosphatemia, are crucial for treatment continuity.
- Further prospective studies are needed to validate these findings and optimize patient selection.
You can read the full article here.


