At the ESMO Breast Cancer Congress 2025, Dr. Suzette Delaloge presented the primary results from the EL1SSAR trial (Late-Breaking Abstract LBA3), a prospective Phase IIIb study evaluating atezolizumab (atezo) plus nab-paclitaxel (nPac) in patients with PD-L1-positive advanced triple-negative breast cancer (aTNBC). This global, single-arm study builds on the success of the IMpassion130 trial and assesses real-world safety and efficacy in a broader population.
What Was the Trial Design and Who Were the Patients in the EL1SSAR Study?
The EL1SSAR study (NCT04148911) enrolled 182 patients with measurable, unresectable locally advanced or metastatic PD-L1+ aTNBC. Eligible participants had not received prior systemic therapy for aTNBC. PD-L1 positivity was defined as ≥1% expression on tumor-infiltrating immune cells using the VENTANA SP142 assay. Unlike IMpassion130, EL1SSAR included patients with stable CNS metastases, ECOG PS 2, and selected autoimmune diseases.
Treatment Regimen
Patients received atezolizumab (840 mg on days 1 and 15) and nab-paclitaxel (100 mg/m² on days 1, 8, and 15) in 28-day cycles, continuing until progression or unacceptable toxicity. The median number of cycles was six, with 7% of patients treated for over three years.
Key Outcomes
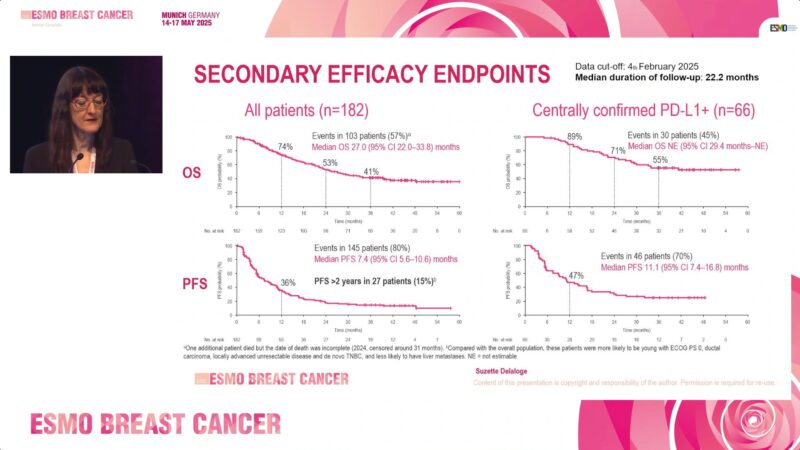
Grade ≥2 immune-mediated adverse events (imAEs) occurred in 12% of patients, and grade ≥3 adverse events in 47%, with no treatment-related deaths. Importantly, outcomes were notably better in patients with centrally confirmed PD-L1+ tumors, highlighting the significance of accurate biomarker testing.
Median progression-free survival (PFS):
- Overall: 7.4 months
- Centrally confirmed PD-L1+: 11.1 months
Median overall survival (OS):
- Overall: 27.0 months
- Centrally confirmed PD-L1+: Not Estimable (NE)
Patients without prior anticancer therapy experienced superior survival (median OS: 33.8 months) compared to those previously treated (OS: 22.3 months), further supporting early use of atezo+nPac in PD-L1+ aTNBC.
Read Full Abstract on ESMO Official Website
What Makes The EL1SSAR Trial Results in PD-L1+ Advanced TNBC So Important?
The EL1SSAR results are consistent with findings from IMpassion130 and reaffirm the clinical benefit of atezolizumab in appropriately selected patients. Notably, only 67% concordance was observed between local and central PD-L1 testing, underscoring the importance of rigorous biomarker assessment in clinical decision-making.
“Suzette Delaloge presents EL1STAR -broadened population for 1L abraxane and atezo (brain Mets, ECOG2, autoimmune diseases). Safety and efficacy very in line with what we expected. 12% G2 greater immune AEs, in central PDL1+, PFS 11.1 Mo”
Erika Hamilton MD, Director of Breast Cancer Research Program at Sarah Cannon Research Institute X
What This Means for Patients?
The EL1SSAR trial confirms that atezolizumab plus nab-paclitaxel offers a meaningful therapeutic option for patients with PD-L1+ advanced TNBC in real-world practice. Central PD-L1 testing remains essential to maximize clinical benefit. These results may influence future diagnostic workflows and treatment strategies for this aggressive breast cancer subtype.
You Can Also Read Final Overall Survival Results from the APHINITY Trial Confirm Long-Term Benefit of Adjuvant Pertuzumab in Early HER2-Positive Breast Cancer by Oncodaily

More updates on ESMO Breast 2025 on OncoDaily.
Written by Aharon Tsaturyan MD


