During ESMO Breast Cancer Congress 2025, Long-term follow-up data from the pivotal Phase III APHINITY trial (NCT01358877), presented today by Dr. Sibylle Loibl, confirm that adjuvant pertuzumab (P) plus trastuzumab (T) and chemotherapy (CT) delivers a statistically significant overall survival (OS) benefit in patients with early-stage HER2-positive breast cancer, especially those at high risk of recurrence.
What Was the APHINITY Trial About?
APHINITY (Adjuvant Pertuzumab and Herceptin IN Initial TherapY) is one of the largest randomized, double-blind, placebo-controlled Phase III trials conducted in the adjuvant treatment of HER2-positive early breast cancer. The study enrolled 4,805 patients with operable, HER2-positive tumors between November 2011 and August 2013 across more than 40 countries.
Patients were randomized in a 1:1 ratio to receive either:
-
Pertuzumab + trastuzumab + chemotherapy (P + T + CT)
-
Placebo + trastuzumab + chemotherapy (Pla + T + CT)
Treatment consisted of one year of anti-HER2 therapy, and chemotherapy regimens could be anthracycline-based or non-anthracycline-based, at the discretion of the treating physician. The study design incorporated stratification by nodal status (node-positive vs. node-negative) and hormone receptor (HR) status (HR-positive vs. HR-negative) to enable predefined subgroup analyses.
The co-primary endpoints were:
-
Invasive disease-free survival (IDFS)
-
Overall survival (OS)
Final Results After 11.3 Years of Follow-Up
At a median follow-up of 11.3 years (clinical cutoff: November 28, 2024), data from 4,804 patients revealed the following: You can read Full Abstract on ESMO
Overall survival (OS)
- 205 deaths (8.5%) in the pertuzumab arm vs. 247 deaths (10.3%) in the placebo arm
- HR = 0.83 (95% CI: 0.69–1.00; p = 0.044)
- 10-year OS rates: 91.6% with pertuzumab vs. 89.8% with placebo (Δ 1.8%)
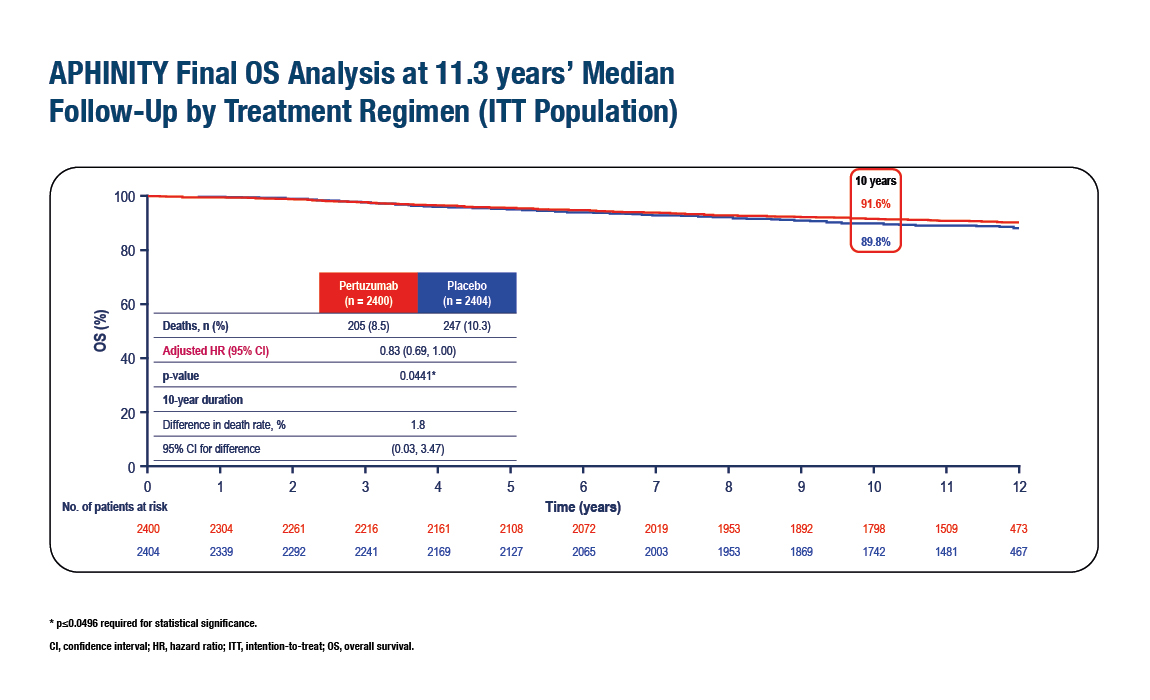
Figure from ESMO Daily Reporter
Subgroup analyses:
- Node-positive: HR = 0.79 (95% CI: 0.64–0.97)
- Node-negative: HR = 0.99 (95% CI: 0.66–1.49)
- Hormone receptor-positive: HR = 0.76 (95% CI: 0.60–0.97)
- Hormone receptor-negative: HR = 0.94 (95% CI: 0.70–1.26)
Invasive disease-free survival (IDFS):
- 12.6% of patients in the pertuzumab group experienced IDFS events vs. 15.8% in the placebo group
- HR = 0.79 (95% CI: 0.68–0.92)
- 10-year IDFS rates: 87.2% vs. 83.8% (Δ 3.4%)
- Benefit driven by reduced distant (6.3% vs. 8.8%) and locoregional relapse (1.5% vs. 2.5%)
Cardiac Safety Remains Acceptable
No new cardiac safety signals were observed over more than a decade of follow-up. The rate of primary cardiac events remained below 1%, even in patients receiving anthracycline-containing regimens, reinforcing the long-term safety profile of pertuzumab.
Why These Final Results Matter
These findings mark the first definitive OS benefit from adjuvant pertuzumab in HER2-positive early breast cancer, particularly among node-positive and hormone receptor-positive subgroups. Earlier data already suggested a durable IDFS advantage; the current results confirm this survival benefit extends over time and is clinically meaningful.
Previous analyses—including a third interim OS analysis at 8.4 years—had hinted at this trend, especially in node-positive cohorts. With the final analysis now confirming statistical significance for OS and reaffirming the magnitude of the IDFS benefit, pertuzumab’s role as a cornerstone of treatment in high-risk HER2-positive early breast cancer is validated.
Key Takeaways
-
First statistically significant OS benefit demonstrated for pertuzumab in early HER2+ breast cancer
-
Greatest benefit seen in node-positive and HR-positive subgroups
-
No survival advantage in node-negative patients—highlighting the need for individualized treatment
-
Cardiac safety remains favorable after 11+ years of follow-up
-
Findings confirm that long-term follow-up is critical to fully understanding the impact of adjuvant therapies
Presented as Late-Breaking Abstract LBA1 at ESMO Breast Cancer 2025, the final APHINITY trial results firmly establish the long-term survival advantage of adding pertuzumab to trastuzumab-based adjuvant therapy in patients with high-risk, HER2-positive early breast cancer. As survivorship improves and de-escalation strategies emerge, these results help define the patients who continue to benefit most from intensive HER2-directed treatment.
More updates on ESMO Breast 2025 on OncoDaily.