DYNAMIC-III trial, presented by Dr. Jeanne Tie at the ESMO Congress 2025, investigated whether post-surgery ctDNA testing could safely guide adjuvant chemotherapy (ACT) in patients with stage III colon cancer. Although ACT is standard in this setting, not all patients derive equal benefit, and many experience unnecessary toxicity. The trial evaluated whether ctDNA-guided treatment de-escalation or escalation could maintain clinical outcomes while reducing chemotherapy exposure and adverse effects, advancing the concept of personalized adjuvant therapy in colorectal cancer.
Background
While adjuvant chemotherapy improves survival in stage III colon cancer, identifying which patients truly benefit remains a clinical challenge. Detectable circulating tumor DNA (ctDNA) after surgery indicates molecular residual disease (MRD) and predicts a high risk of recurrence, whereas ctDNA negativity suggests minimal residual disease and a low recurrence risk. The DYNAMIC-III study was designed to test whether ctDNA-guided management could optimize treatment intensity—de-escalating therapy in low-risk (ctDNA-negative) patients and maintaining or escalating therapy in those at high molecular risk (ctDNA-positive).
Methods
This multicenter, randomized, phase II/III trial enrolled patients with stage III colon cancer approximately 5–6 weeks post-surgery. Participants underwent tumor-informed ctDNA testing and were randomized 1:1 to either ctDNA-guided management or standard care.
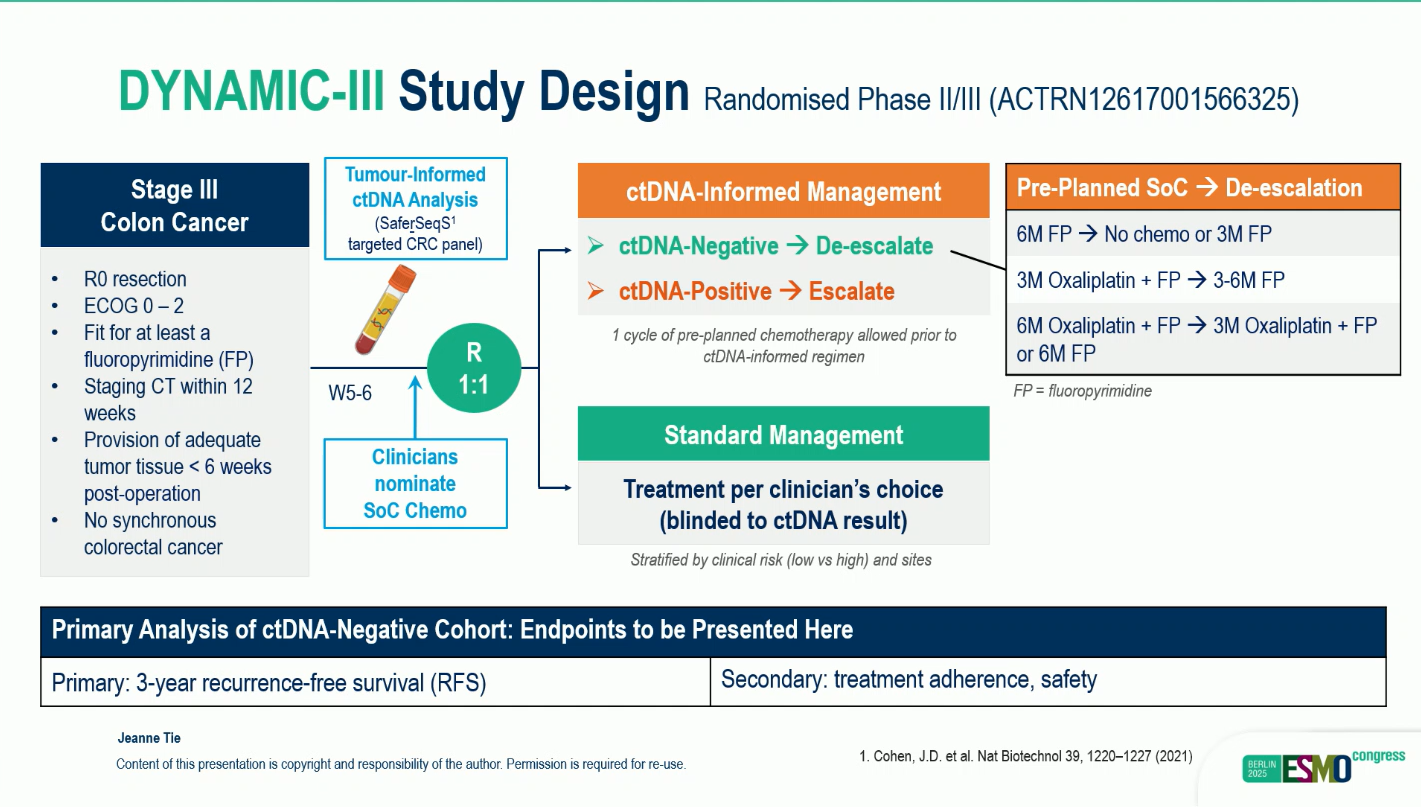
In the ctDNA-guided arm, ctDNA-negative patients received de-escalated therapy, which could include shortened chemotherapy duration (6 to 3 months), reduced regimen intensity (from doublet to single-agent fluoropyrimidine), or observation.
Primary endpoint: 3-year recurrence-free survival (RFS), with a non-inferiority (NI) margin of 7.5% and 80% power at a one-sided 97.5% confidence interval.
Results
Out of 968 evaluable patients, 702 (72.5%) were ctDNA-negative, with 353 assigned to ctDNA-guided management and 349 to standard care.
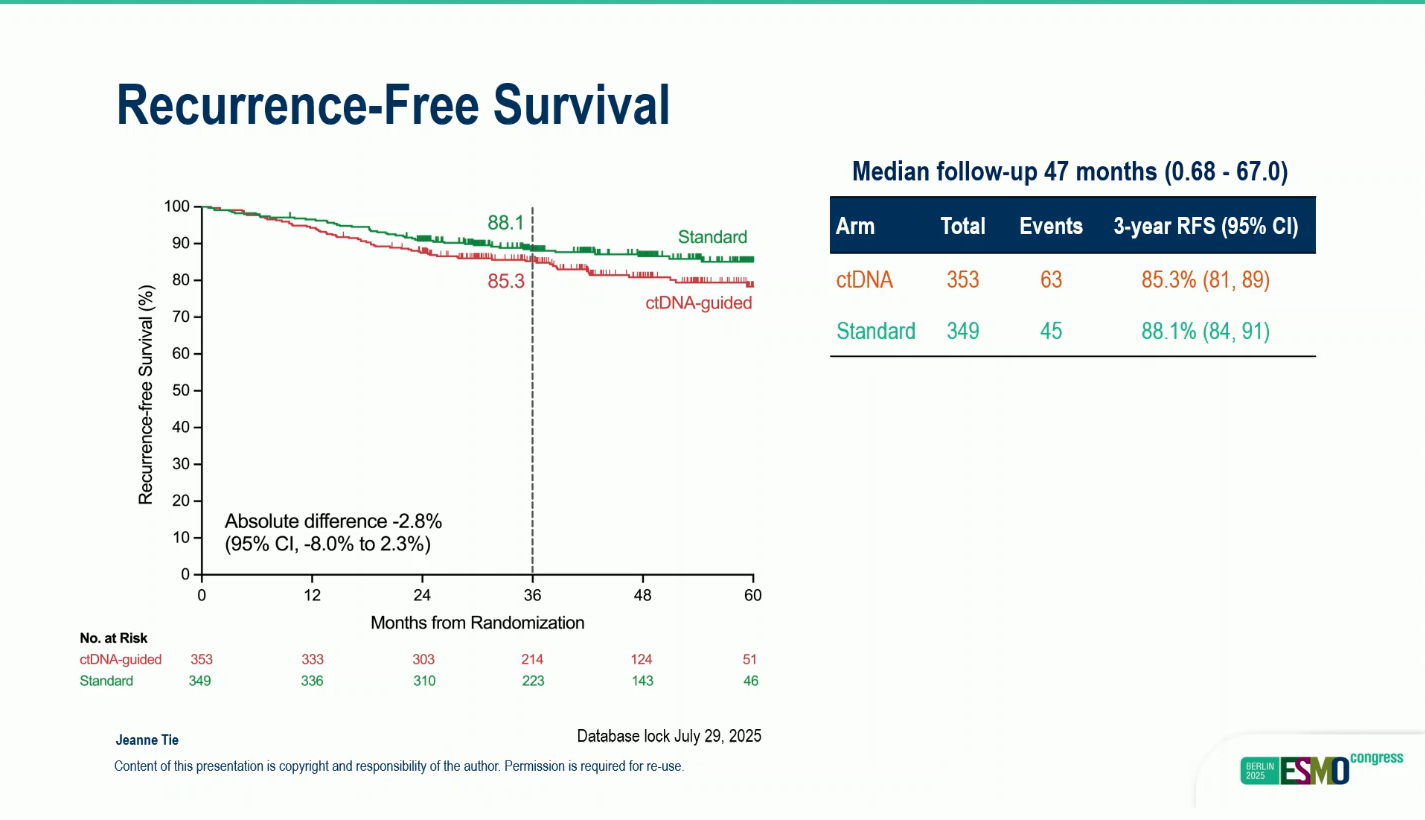
- Median follow-up: 47 months
- 3-years RFS:85,3% ctDNA arm vs 88.1% standart arm
- Oxaliplatin use: 34.8% (ctDNA-guided) vs 88.6% (standard), P < 0.001
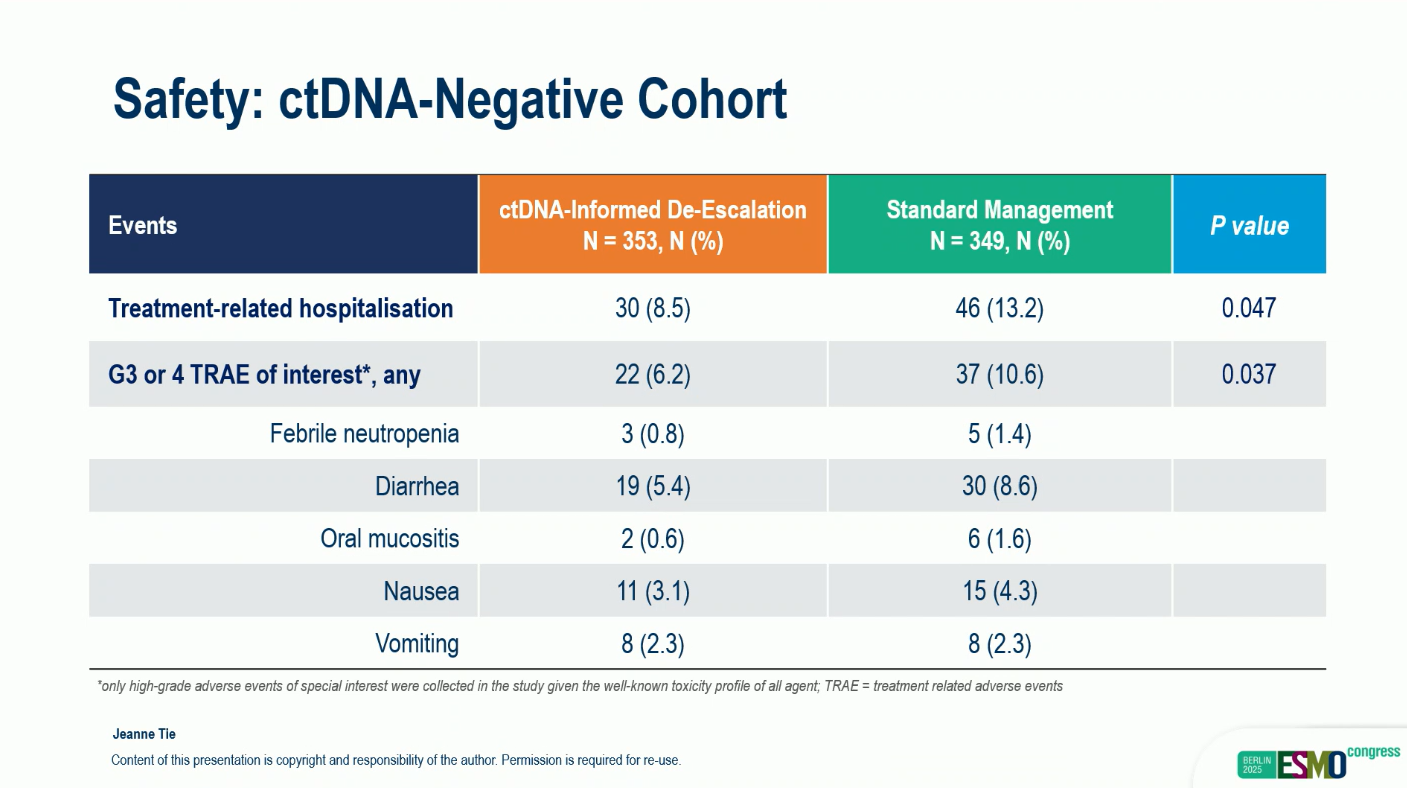
- Grade ≥3 adverse events: 6.2% vs 10.6%, P = 0.037
- Treatment-related hospitalizations: 8.5% vs 13.2%, P = 0.048
While ctDNA-guided management substantially reduced chemotherapy exposure and toxicity, non-inferiority in 3-year RFS was not achieved (85.3% vs 88.1%; difference –2.8%; 97.5% lower CI –8.0%).
However, in clinically low-risk tumors (T1–3N1), de-escalation outcomes were comparable to standard therapy (3-year RFS 91.0% vs 93.2%; difference –2.2%; 97.5% lower CI –7.2%), suggesting this subgroup could safely benefit from less intensive treatment.
Conclusions
The DYNAMIC-III trial demonstrated that patients with negative post-surgery ctDNA have a low recurrence risk, supporting ctDNA as a robust biomarker for tailoring adjuvant therapy. Although overall non-inferiority was not established, ctDNA-guided de-escalation markedly reduced oxaliplatin exposure and toxicity, maintaining favorable survival outcomes—especially in clinically low-risk patients. These findings reinforce the feasibility of ctDNA-based personalized adjuvant strategies in stage III colon cancer and pave the way for further precision oncology trials.
You can read the full abstract here.