On August 6, 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Dordaviprone for the treatment of adult and pediatric patients aged 1 year and older with diffuse midline glioma (DMG) harboring an H3 K27M mutation who have experienced disease progression following prior therapies. This marks a historic milestone in neuro-oncology, as Dordaviprone becomes the first FDA-approved systemic therapy specifically targeting H3 K27M-mutant diffuse midline glioma, a rare and aggressive brain tumor primarily affecting children and young adults.
Clinical Trials and Population Studied
The FDA’s decision was based on an integrated efficacy analysis of 50 patients treated across five early-phase clinical trials conducted in the United States. All were non-randomized, open-label studies, enrolling patients with progressive and measurable H3 K27M-mutant diffuse midline glioma, who had received prior standard therapy (typically radiation) and had no signs of cerebrospinal fluid (CSF) dissemination. Each study had a unique role in evaluating safety, dosing, pharmacokinetics, and ultimately, antitumor activity of single-agent dordaviprone.
- ONC006 – Phase 2 dose-escalation study that first evaluated the safety, tolerability, and pharmacokinetics of dordaviprone in adult patients with high-grade glioma. It identified the recommended dose for further testing.
- ONC013 – Phase 2 trial, included both adults and children to assess early signs of antitumor activity and further define safety in a mixed-age population with H3 K27M mutations.
- ONC014 – Pediatric-focused Phase 1 trial designed to determine appropriate weight-based dosing and evaluate tolerability and exposure in children with recurrent diffuse midline glioma.
- ONC016 – Expanded access study, contributed the primary data for regulatory evaluation. Responses were assessed by blinded independent review using RANO 2.0 criteria.
- ONC018 – Long-term follow-up study monitoring durability of response, late safety signals, and ongoing disease control in patients previously treated with dordaviprone.
Eligible patients were those with measurable and progressive H3 K27M-mutant diffuse midline glioma, who had completed radiation therapy at least 90 days prior, had adequate performance status (KPS or LPS ≥60), and were either off corticosteroids or on a decreasing dose. The study population included both adults and children, and all participants were treated with single-agent dordaviprone. Importantly, patients with diffuse intrinsic pontine glioma (DIPG), spinal cord gliomas, cerebrospinal fluid (CSF) dissemination, or atypical tumor histologies were excluded.
Efficacy Results
Dordaviprone demonstrated meaningful antitumor activity in a population with few other treatment options. The overall response rate (ORR) was 22% (95% CI: 12–36), as assessed by blinded independent central review (BICR) using RANO 2.0 criteria. The median duration of response (DOR) was 10.3 months (95% CI: 7.3–15.2), reflecting durable clinical benefit in responders.
Among the 11 patients who achieved an objective response, 73% had responses lasting six months or longer, and 27% maintained responses for over a year. These findings, while preliminary, highlight the potential of dordaviprone to meaningfully alter the course of this devastating disease in a subset of patients.
Safety Profile and Warnings
Modeyso was generally well-tolerated, although the prescribing information includes key warnings for hypersensitivity reactions, QTc interval prolongation, and embryo-fetal toxicity. Due to the risk of cardiac side effects, ECG monitoring may be advised in patients with known risk factors or those taking other QT-prolonging medications. Reproductive counseling is also recommended for patients of childbearing potential.
Overall, the safety profile observed in clinical trials supports the use of dordaviprone in appropriately selected patients under careful clinical monitoring.
Read more on FDA Official Website.
Dosing and Administration
For adult patients, the recommended dose of dordaviprone is 625 mg orally once weekly. In pediatric patients, dosing is adjusted based on body weight, with specific guidance provided in the full prescribing information. Modeyso is administered orally and does not require intravenous infusion, which may be especially beneficial for pediatric populations.

You can also read about Glioblastoma: Causes, Symptoms, Diagnosis, Treatment, and Latest Advances in 2025 on OncoDaily.
Understanding Diffuse Midline Glioma (DMG)
Diffuse midline glioma (DMG) is a highly aggressive and fatal type of brain tumor that arises in midline structures of the central nervous system, such as the brainstem (pons), thalamus, and spinal cord. DMG primarily affects children and young adults but can also occur in older patients. These tumors are diffuse, meaning they infiltrate healthy brain tissue, making surgical removal nearly impossible.
In 2016, the World Health Organization (WHO) reclassified these tumors as a distinct entity due to their unique genetic profile and clinical behavior. Median overall survival is less than one year from diagnosis.
The defining mutation in DMG is the H3 K27M mutation. Other common genetic changes include mutations in TP53, ACVR1, and overexpression or alterations in PDGFRA, EGFR, and EZHIP. These genetic alterations influence tumor behavior and are key to diagnosis and emerging targeted treatments. Treatment typically includes radiation therapy, but the response is limited and recurrence is common. No targeted therapies were approved for this population—until the recent FDA accelerated approval of dordaviprone in August 2025.
What Is the H3 K27M Mutation?
The H3 K27M mutation is a hallmark genetic alteration found in most diffuse midline gliomas. It occurs in genes encoding the histone H3 family—most commonly H3F3A or HIST1H3B/C—leading to a substitution of lysine (K) with methionine (M) at position 27 of histone H3. This mutation disrupts normal gene expression by blocking trimethylation of H3K27, a key epigenetic silencing mechanism.
As a result, tumor cells exhibit abnormal chromatin structure, leading to uncontrolled cell growth and resistance to standard therapies. The presence of the H3 K27M mutation has significant diagnostic and prognostic value, and it is now part of the WHO classification: “Diffuse midline glioma, H3 K27-altered”.
This mutation defines a biologically distinct subgroup of high-grade gliomas, and its identification is essential for diagnosis, prognostication, and now—targeted drug development, such as the use of dordaviprone, which specifically targets the mutant pathway.
What is Dordaviprone and how does it work?
Dordaviprone, marked under the brand name Modeyso, is a first-in-class protease activator, a novel class of agents that enhances intracellular protease activity, potentially promoting tumor cell death by degrading oncogenic proteins. Although the precise mechanism in H3 K27M-mutant glioma is still being studied, preclinical data suggest that dordaviprone may target the epigenetic and transcriptional vulnerabilities conferred by the H3 K27M mutation.
The drug was developed by Jazz Pharmaceuticals, a biopharmaceutical company focused on rare diseases and oncology. Dordaviprone reflects Jazz’s growing pipeline in neuro-oncology and marks a significant step forward in the field.
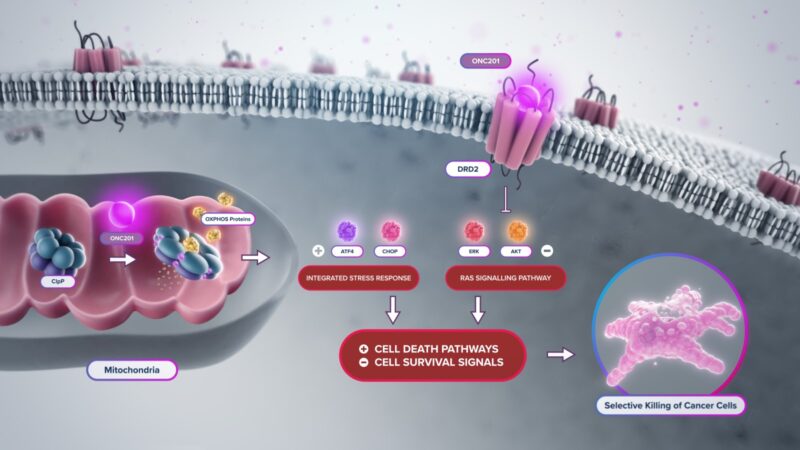
Source: Chimerix Official Website, a part of Jazz Pharmaceuticals
Future Outlook
The accelerated approval of Dordaviprone is contingent on confirmatory evidence of clinical benefit. Ongoing and planned studies are expected to further evaluate Modeyso’s impact on overall survival, quality of life, and long-term safety in children and adults with H3 K27M-mutant gliomas.
For now, this decision represents a major therapeutic advance, offering hope to families and clinicians navigating the challenges of recurrent diffuse midline glioma. As the first targeted systemic therapy approved for this population, dordaviprone could help redefine treatment strategies in the years ahead.


