Glioblastoma, also known as glioblastoma multiforme (GBM), is the most aggressive and common primary malignant brain tumor in adults. Arising from astrocytes, star-shaped glial cells that support nerve cells—glioblastomas are classified as grade IV astrocytomas by the World Health Organization (WHO), indicating the highest level of malignancy. These tumors are known for their rapid growth, invasiveness, and resistance to conventional therapies.
Types of Glioblastoma
Glioblastoma (GBM) is a highly heterogeneous disease with varying clinical behaviors, molecular features, and prognoses. Classification is crucial for determining prognosis and tailoring personalized therapy.
Primary Glioblastoma
Primary GBM arises without evidence of a preexisting lower-grade lesion and is the most prevalent form, comprising over 90% of cases. It primarily affects older adults, with a median age of onset around 60–70 years. This type exhibits rapid onset and progression, often presenting with headaches, seizures, or cognitive decline. Molecularly, primary GBMs are typically IDH-wildtype, harboring EGFR amplification, PTEN deletions, and TERT promoter mutations. These alterations promote uncontrolled cell proliferation and resistance to apoptosis (Louis et al., 2021). Primary GBM is considered more aggressive than secondary GBM and is often associated with poorer survival outcomes.
Secondary Glioblastoma
Secondary GBMs develop from previously diagnosed lower-grade astrocytomas (WHO grade II or III), usually over a period of years. They are more common in younger patients, with a median onset in the fourth to fifth decades of life. Histologically indistinguishable from primary GBM, secondary GBMs are defined by distinct genetic alterations, especially IDH1 or IDH2 mutations, which are early events in tumor development. TP53 mutations and ATRX loss are also characteristic. The presence of IDH mutation is associated with better response to therapy and improved survival, making molecular testing critical for diagnosis and treatment planning (Weller et al., 2021).
Glioblastoma with H3F3A Mutation
These glioblastomas are categorized by specific mutations in histone genes, particularly H3 K27M and H3 G34R/V. H3 K27M-mutant tumors are now formally classified as “diffuse midline gliomas” and are typically found in midline structures such as the thalamus, pons, or spinal cord. They predominantly affect children and young adults and are associated with poor outcomes. H3 G34R/V mutations, found in cerebral hemispheres, are associated with aggressive behavior and are more prevalent in adolescents and young adults. These tumors demonstrate high-grade histological features and are often resistant to conventional therapy (Louis et al., 2021).
Epithelioid Glioblastoma
This rare subtype primarily affects children and young adults and is distinguished histologically by large, round cells with prominent nucleoli and a rich cytoplasm resembling epithelial cells. It may exhibit rhabdoid features and has a high mitotic index. Importantly, a subset of these tumors harbor BRAF V600E mutations, which may be targeted with specific inhibitors. Epithelioid GBMs are known for rapid progression and high rates of leptomeningeal dissemination, which contributes to a poor prognosis (Weller et al., 2021).

Read More About Glioblastoma in Children on Oncodaily
Giant Cell Glioblastoma
A rare variant, giant cell glioblastoma accounts for about 1% of GBMs and is marked by the presence of multinucleated giant cells and a high degree of cytological atypia. This subtype occurs more frequently in younger patients and has a relatively more favorable prognosis. Genetic studies often reveal TP53 mutations, but IDH mutations are rare. Its indolent course and distinct histopathology may aid in differentiation and influence treatment approaches (Louis et al., 2021).
Gliosarcoma
Gliosarcoma is a biphasic tumor composed of both glial and mesenchymal (sarcomatous) elements, representing a variant of GBM. It occurs in approximately 2% of cases and typically affects adults. This tumor often presents as a firm, well-demarcated mass and can mimic meningioma radiologically. The sarcomatous component may demonstrate features of fibrosarcoma or osteosarcoma. Although managed similarly to conventional GBM, gliosarcomas may show unique patterns of spread and recurrence. The prognosis is poor, similar to primary GBM, although some studies suggest distinct biological behavior (Weller et al., 2021).
Causes of Glioblastoma
Glioblastoma, the most aggressive primary brain tumor in adults, arises from complex interactions between genetic, molecular, and environmental factors. While most cases are sporadic, advances in molecular profiling have uncovered several underlying mechanisms.Genetically, glioblastomas frequently harbor mutations in key regulatory genes:
- TP53, a tumor suppressor gene, is commonly mutated, leading to a loss of cell cycle control (Weller et al., 2021).
- EGFR (epidermal growth factor receptor) amplification or mutation is seen in nearly 50% of glioblastomas, promoting tumor growth through the PI3K/AKT and RAS/MAPK signaling pathways (Louis et al., 2021).
- PTEN mutations contribute to unchecked cell proliferation by disrupting negative regulation of the PI3K pathway (Louis et al., 2021).
- Mutations in IDH1/IDH2 are typically found in secondary glioblastomas and are associated with a better prognosis (Weller et al., 2021).
Epigenetic changes, such as MGMT promoter methylation, are also significant. This silencing of the DNA repair enzyme MGMT enhances tumor response to temozolomide, an alkylating chemotherapeutic agent (Weller et al., 2021).
Environmental exposures are not strongly linked to glioblastoma, with the notable exception of ionizing radiation, particularly therapeutic radiation during childhood (Louis et al., 2021). There is insufficient evidence to support a causal role for mobile phones, occupational chemicals, or dietary factors.
Epidemiologically, glioblastoma occurs more commonly in older adults, typically between 65 and 75 years of age, and is slightly more prevalent in males than females, possibly due to hormonal or chromosomal influences (Louis et al., 2021).Though rare, hereditary cancer syndromes can increase the risk:
- Li-Fraumeni syndrome (TP53 mutations)
- Neurofibromatosis type 1 (NF1 mutations)
- Turcot syndrome (APC or mismatch repair gene mutations)
These inherited disorders underline the role of germline mutations in glioblastoma pathogenesis in select cases (Weller et al., 2021).
Symptoms of Glioblastoma
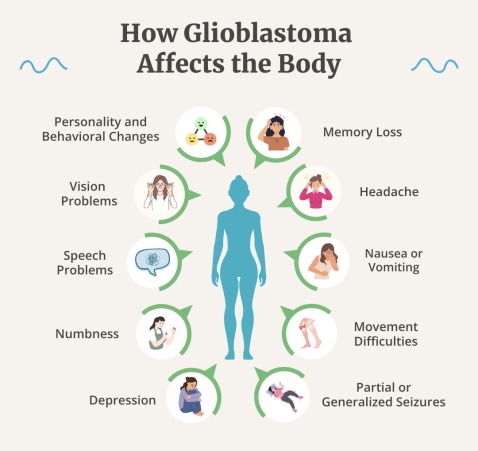
Glioblastoma presents with a wide range of neurological symptoms that often reflect the tumor’s location, size, and the rate at which it grows. Because glioblastoma is an aggressive and infiltrative brain tumor, its manifestations may appear rapidly and worsen quickly over days to weeks.
The most common symptom is headache, typically caused by increased intracranial pressure due to tumor growth or associated edema. These headaches often worsen in the morning or with changes in posture. Seizures are another frequent presenting feature, especially in younger patients or when the tumor involves the cerebral cortex. They may occur as focal motor seizures, generalized tonic-clonic seizures, or even subtle changes in behavior.
Cognitive and personality changes are also notable. Patients may experience memory deficits, impaired judgment, confusion, or emotional instability. These symptoms can be mistaken for psychiatric or neurodegenerative disorders, leading to delays in diagnosis (Louis et al., 2021).
Motor weakness, particularly hemiparesis, occurs when the tumor invades or compresses motor pathways. This can lead to difficulty walking, grasping objects, or performing routine tasks. Speech disturbances (aphasia) are seen when glioblastoma affects the dominant hemisphere’s language areas. Patients may have trouble finding words, understanding speech, or forming coherent sentences.
Visual disturbances such as blurred vision, visual field cuts, or even visual hallucinations may occur when the occipital lobe or optic pathways are involved. Tumors in the posterior fossa can cause nausea, vomiting, and gait ataxia due to pressure on the cerebellum or obstruction of cerebrospinal fluid pathways, leading to hydrocephalus.
In cases of large tumors or those located near critical structures like the brainstem, symptoms may rapidly progress to altered consciousness, coma, or even herniation, which is life-threatening. Because glioblastoma often causes vasogenic edema, symptoms can also vary with steroid administration, which temporarily reduces swelling and improves neurological status (Weller et al., 2021).
Diagnosis of Glioblastoma
Diagnosing glioblastoma requires a combination of clinical evaluation, neuroimaging, histopathological analysis, and increasingly, molecular profiling. Due to the tumor’s aggressive growth, patients often present with rapidly progressive neurological symptoms, prompting urgent medical evaluation.
Neuroimaging is the cornerstone of initial diagnosis. Magnetic resonance imaging (MRI) with contrast is the preferred modality due to its superior resolution and ability to characterize brain tumors. Glioblastomas typically appear as irregular, ring-enhancing lesions with central necrosis and surrounding vasogenic edema. T1-weighted images show the contrast-enhancing tumor margins, while T2-weighted and FLAIR sequences highlight the extent of edema and infiltrative spread (Weller et al., 2021).
Diffusion-weighted imaging (DWI) and perfusion MRI can help differentiate glioblastoma from abscesses or other brain lesions. Positron emission tomography (PET) and magnetic resonance spectroscopy (MRS) may be used to assess metabolic activity and distinguish high-grade tumors from low-grade gliomas or treatment effects.
Histopathological confirmation is mandatory for a definitive diagnosis. This is achieved through stereotactic biopsy or surgical resection. Histologically, glioblastomas exhibit hypercellularity, nuclear atypia, mitotic figures, microvascular proliferation, and necrosis, hallmarks of a WHO grade 4 astrocytoma (Louis et al., 2021).
Molecular diagnostics play an increasingly critical role. Testing for mutations in the isocitrate dehydrogenase (IDH1/2) gene is essential, as IDH-wildtype glioblastomas have a poorer prognosis and different biology compared to IDH-mutant astrocytomas. Other key markers include O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status, which predicts response to alkylating agents like temozolomide, and epidermal growth factor receptor (EGFR) amplification, particularly the EGFRvIII variant (Weller et al., 2021).
The 2021 WHO classification of CNS tumors has refined glioblastoma criteria, requiring IDH-wildtype status and the presence of specific histological or molecular features such as TERT promoter mutations, EGFR amplification, or combinations gain of chromosome 7 and loss of chromosome 10 (7+/10−) (Louis et al., 2021).
Prognosis of Glioblastoma
Glioblastoma (GBM) carries a poor prognosis due to its aggressive biology, diffuse infiltration, and resistance to conventional therapies. It remains one of the most lethal primary brain tumors in adults, with median overall survival typically ranging from 12 to 15 months even with optimal treatment, including surgery, radiotherapy, and chemotherapy (Stupp et al., 2005).
Key prognostic factors include age, performance status, extent of surgical resection, molecular markers, and treatment response. Younger patients (<60 years) and those with a high Karnofsky Performance Status (KPS ≥ 70) generally fare better. Gross total resection, when feasible, is associated with prolonged survival compared to partial resection or biopsy alone (Weller et al., 2021). Molecular markers have a profound impact on prognosis. The IDH mutation status is the most important molecular prognostic factor, IDH-mutant glioblastomas, though rare in adults, show significantly improved survival, with median survival extending beyond 3 years in many cases. In contrast, IDH-wildtype GBM is more aggressive and associated with poorer outcomes (Louis et al., 2021).
MGMT promoter methylation is another favorable prognostic marker. Patients with MGMT-methylated tumors show a better response to alkylating chemotherapy, such as temozolomide, and demonstrate improved overall survival (Hegi et al., 2005). In clinical trials, MGMT-methylated patients had a median survival of over 20 months compared to less than 12 months in unmethylated cases. Despite advances in therapy, the 5-year survival rate remains below 10%, with most patients succumbing to disease progression. Recurrence is almost universal, often within 6–9 months of initial therapy, and treatment options at recurrence are limited.
Emerging therapies, including tumor-treating fields (TTFields), immunotherapy, and targeted agents, have shown modest improvements in progression-free survival, but breakthroughs that significantly alter the prognosis are still under investigation (Weller et al., 2021).
Treatment of Glioblastoma
The treatment of glioblastoma (GBM) is multidisciplinary and aims to maximize tumor control while preserving neurological function. Despite aggressive treatment, glioblastoma remains incurable, and therapy focuses on extending survival and improving quality of life.
The standard first-line treatment consists of maximal safe surgical resection followed by concurrent radiotherapy with temozolomide chemotherapy, and then adjuvant temozolomide. This protocol, known as the Stupp regimen, remains the cornerstone of glioblastoma management and has been shown to improve median survival from 12.1 months with radiotherapy alone to 14.6 months with the addition of temozolomide (Stupp et al., 2005). Surgical resection aims to reduce tumor burden, relieve symptoms, and obtain tissue for histopathologic and molecular diagnosis. Gross total resection is associated with improved outcomes, but complete removal is rarely possible due to the tumor’s invasive nature (Weller et al., 2021).
Radiotherapy is delivered postoperatively, typically as 60 Gy in 30 fractions over six weeks. It plays a vital role in local tumor control. Advanced techniques such as intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) help minimize damage to healthy brain tissue.

Read More About Radiotherapy for Brain Tumor on Oncodaily
Chemotherapy with temozolomide (TMZ), an oral alkylating agent, is administered daily during radiotherapy and continued as adjuvant therapy for six cycles or more. The efficacy of TMZ is significantly influenced by the MGMT promoter methylation status. Patients with MGMT-methylated tumors show better response rates and improved overall survival compared to those without methylation (Hegi et al., 2005).
Tumor Treating Fields (TTFields), a non-invasive, wearable device that delivers alternating electric fields to disrupt cancer cell division, has been incorporated into treatment for newly diagnosed glioblastoma. The EF-14 trial demonstrated that TTFields combined with TMZ significantly extended median overall survival to 20.9 months compared to 16 months with TMZ alone (Stupp et al., 2017).
Treatment for recurrent glioblastoma remains challenging. Options include re-operation, re-irradiation, and systemic therapies such as bevacizumab, an anti-VEGF monoclonal antibody, which can reduce edema and improve symptoms though it does not significantly extend survival (Weller et al., 2021). Other salvage therapies include nitrosoureas (e.g., lomustine), irinotecan, and participation in clinical trials.
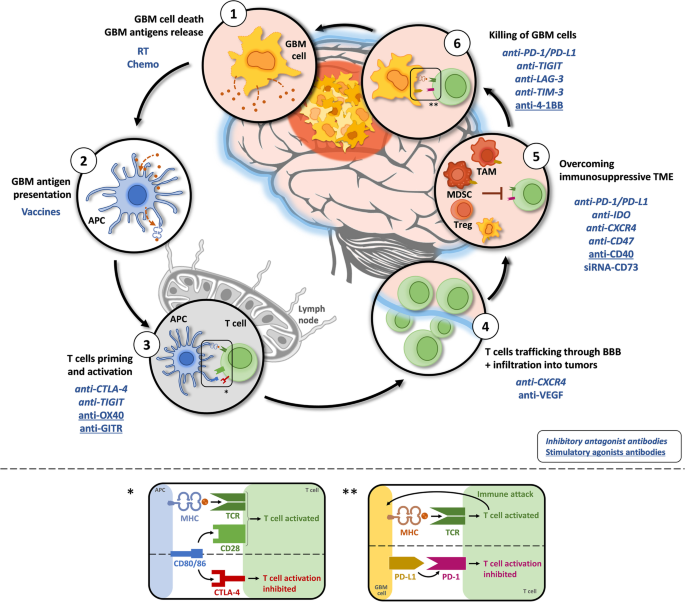
Immunotherapy and targeted therapies have been extensively investigated. Checkpoint inhibitors (e.g., nivolumab) have shown limited efficacy due to the immunosuppressive tumor microenvironment of GBM. Trials exploring vaccines, CAR-T cells, and oncolytic viruses are ongoing, showing promise in selected patient subsets (Lim et al., 2018).
Read More About Immunotherapy for Brain Cancer on Oncodaily
Personalized treatment based on molecular features such as IDH mutation, TERT promoter mutation, and EGFR amplification is gaining momentum. IDH-mutant glioblastomas tend to have a better prognosis and may respond differently to therapies compared to IDH-wildtype tumors.
Latest 2025 Advances in Glioblastoma Treatment
As of 2025, significant advances in glioblastoma multiforme (GBM) treatment have emerged from innovations in immunotherapy, molecular targeting, and cellular-based therapies. These developments aim to overcome resistance to standard treatments and improve long-term outcomes in a disease with historically limited survival.
Immunotherapy and Vaccine Strategies
Checkpoint inhibitors, such as nivolumab and pembrolizumab, have continued to show limited efficacy as monotherapy in unselected GBM populations. However, recent trials suggest that combining PD-1 inhibitors with personalized neoantigen vaccines may enhance immune response in select patients (Lim et al., 2025). This combinatorial approach has demonstrated extended progression-free survival in patients with MGMT-methylated and hypermutated tumors.
Oncolytic Viral Therapy
The use of engineered oncolytic viruses, such as DNX-2401 (Delta-24-RGD), has progressed to Phase III trials. These viruses selectively replicate in tumor cells and stimulate an anti-tumor immune response. A 2025 multicenter study showed that combining DNX-2401 with immune checkpoint inhibitors produced durable responses in recurrent GBM patients (Sampson et al., 2025).
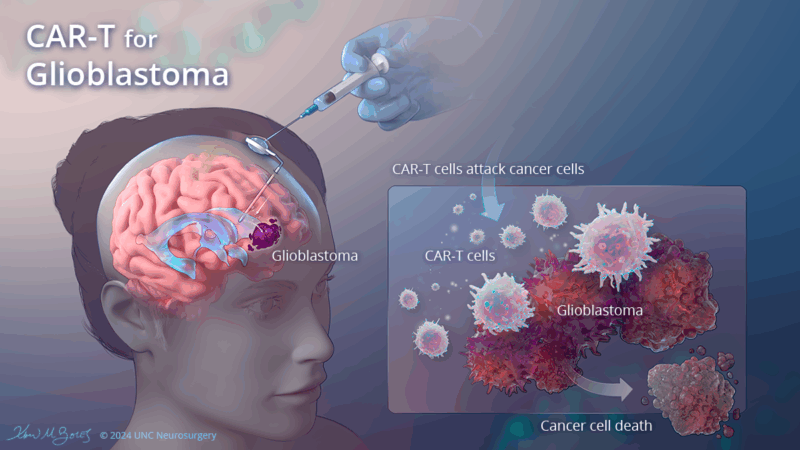
CAR-T Cell Therapy
Chimeric antigen receptor T-cell (CAR-T) therapy targeting EGFRvIII and IL13Rα2 has gained traction. In 2025, a modified form of CAR-T therapy with regional delivery directly into the tumor cavity showed enhanced persistence of T cells and improved tumor control in early-phase trials (Brown et al., 2025). The intraventricular administration technique appears particularly promising for multifocal or recurrent GBM.
Tumor Treating Fields (TTFields) + Combination Regimens
TTFields continue to be part of standard care, but newer trials are combining this technology with agents like abemaciclib (a CDK4/6 inhibitor). Preliminary 2025 data suggest a synergistic anti-proliferative effect, improving progression-free survival by an additional 3–4 months compared to TTFields alone in newly diagnosed GBM (Stupp et al., 2025).
Epigenetic Therapies and IDH-Mutant Targeting
For IDH-mutant glioblastomas, which behave more indolently, novel agents such as IDH1 inhibitors (ivosidenib) are being evaluated. A 2025 update from a randomized Phase II trial reported prolonged median survival beyond 30 months in patients receiving these agents, particularly in combination with radiotherapy and temozolomide (Weller et al., 2025).

Read More About Ivosidenib on Oncodaily
Liquid Biopsy for Real-Time Monitoring
Technological advances have enabled the detection of circulating tumor DNA (ctDNA) and extracellular vesicles in cerebrospinal fluid and blood. These liquid biopsies, now being tested in clinical trials, allow real-time tracking of tumor evolution and treatment resistance (Zhou et al., 2025).
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
FAQ
What is glioblastoma?
Glioblastoma is the most aggressive type of primary brain tumor in adults, classified as a grade IV astrocytoma.
What causes glioblastoma?
The exact cause is unknown, but it is associated with genetic mutations, age, male gender, and prior radiation exposure to the brain.
What are the symptoms of glioblastoma?
Common symptoms include headaches, seizures, memory loss, personality changes, nausea, and focal neurological deficits depending on tumor location.
How is glioblastoma diagnosed?
Diagnosis involves MRI imaging followed by a biopsy or surgical resection for histological and molecular analysis.
What is the standard treatment for glioblastoma?
Standard therapy includes surgical resection, radiotherapy, and concurrent plus adjuvant temozolomide chemotherapy.
Is glioblastoma curable?
Currently, glioblastoma is not curable. Most treatments aim to extend survival and improve quality of life.
What is the typical survival time after diagnosis?
Median overall survival is about 12 to 15 months with standard treatment; however, some patients live longer with favorable prognostic factors.
Do molecular markers affect glioblastoma treatment?
Yes, IDH mutation and MGMT promoter methylation are key molecular markers that influence treatment response and prognosis.
Are there any new treatments available in 2025?
Emerging therapies include tumor-treating fields (TTF), personalized vaccines, CAR T-cell therapy, and trials targeting EGFR, CD73, and PD-L1.
Can glioblastoma recur after treatment?
Yes, recurrence is almost universal, typically occurring within 6 to 9 months of initial therapy.


