A study on dMMR tumors, published in the New England Journal of Medicine by Andrea Cercek, M.D., Michael B. Foote, M.D., Benoit Rousseau, M.D., Ph.D., and colleagues, demonstrates the efficacy of nonoperative management for patients with early-stage dMMR solid tumors. The researchers showed that neoadjuvant therapy using the PD-1 inhibitor dostarlimab achieved a remarkable clinical complete response rate, potentially allowing organ preservation and eliminating the need for surgery in a significant majority of patients. These groundbreaking findings could transform treatment approaches for various cancer types characterized by dMMR status.
Title: Nonoperative Management of Mismatch Repair-Deficient Tumors
Authors: Andrea Cercek, M.D., Michael B. Foote, M.D., Benoit Rousseau, M.D., Ph.D., J. Joshua Smith, M.D., Ph.D., Jinru Shia, M.D., Jenna Sinopoli, N.P., Jill Weiss, B.S., Melissa Lumish, M.D. Lindsay Temple, B.A., Miteshkumar Patel, M.S., Callahan Wilde, B.A., Leonard B. Saltz, M.D., Guillem Argiles, M.D., ZsofiaStadler, M.D., Oliver Artz, Ph.D., Steven Maron, M.D., Geoffrey Ku, M.D., Ping Gu, M.D., Yelena Y. Janjigian, M.D., Daniela Molena, M.D., Gopa Iyer, M.D., Jonathan Coleman, M.D., Wassim Abida, M.D., SethCohen, M.D., Kevin Soares, M.D., Mark Schattner, M.D., Vivian E. Strong, M.D., Rona Yaeger, M.D., PhilipPaty, M.D., Marina Shcherba, M.D., Ryan Sugarman, M.D., Paul B. Romesser, M.D., Alice Zervoudakis, M.D., Avni Desai, M.D., Neil H. Segal, M.D., Ph.D., Imane El Dika, M.D., Maria Widmar, M.D., Iris Wei, M.D., Emmanouil Pappou, M.D., Ph.D., Gerard Fumo, M.D., Santiago Aparo, M.D., Mithat Gonen, M.D., Marc Gollub, M.D., Vetri S. Jayaprakasham, M.B., B.S., Tae-Hyung Kim, M.D., Julio Garcia Aguilar, M.D., Ph.D., Martin Weiser, M.D., and Luis A. Diaz, Jr., M.D.
Published in The New England Journal of Medicine on April 2025
Background
Surgical resection has traditionally been the primary curative option for early-stage solid tumors. While nonoperative management approaches using radiation and chemotherapy have been developed for specific cancers, this study explored extending immunotherapy-based organ preservation to all dMMR tumors, regardless of site.
Previous research established that dMMR tumors, characterized by deficiencies in the DNA mismatch repair system, show exceptional sensitivity to immune checkpoint blockade. This led to the first tumor-agnostic FDA approval for metastatic dMMR cancers. The current study builds on earlier findings in rectal cancer to determine if this approach could be universally applied to all early-stage dMMR solid tumors.

Methods and Trial Design
This phase 2 trial enrolled patients with stage I, II, or III dMMR solid tumors amenable to curative-intent surgery at Memorial Sloan Kettering Cancer Center, Hartford HealthCare, and Baptist Health Miami Cancer Institute. Patients received intravenous dostarlimab (500 mg) every 3 weeks for 6 months (9 cycles total).
- Cohort 1: dMMR locally advanced rectal cancer (primary cohort).
- Cohort 2: dMMR nonrectal solid tumors (esophagogastric, colon, hepatobiliary, genitourinary, gynecologic).
Clinical complete response (CCR) was assessed through imaging, endoscopy, biopsy, and circulating tumor DNA (ctDNA) analysis. Patients with CCR could opt for nonoperative management, while patients showing residual disease underwent standard surgical resection.
Primary Endpoints
- Sustained CCR at 12 months (Cohort 1)
- Recurrence-free survival and safety (both cohorts)
Key Results
117 patients were included in the analysis (7 excluded from 124 enrolled). Of these, 103 completed treatment, and 14 were still receiving treatment at data cutoff. The majority (64%) had evidence of lymph node involvement on imaging, and 44% had pathogenic germline variants in mismatch repair genes.
Clinical Complete Response
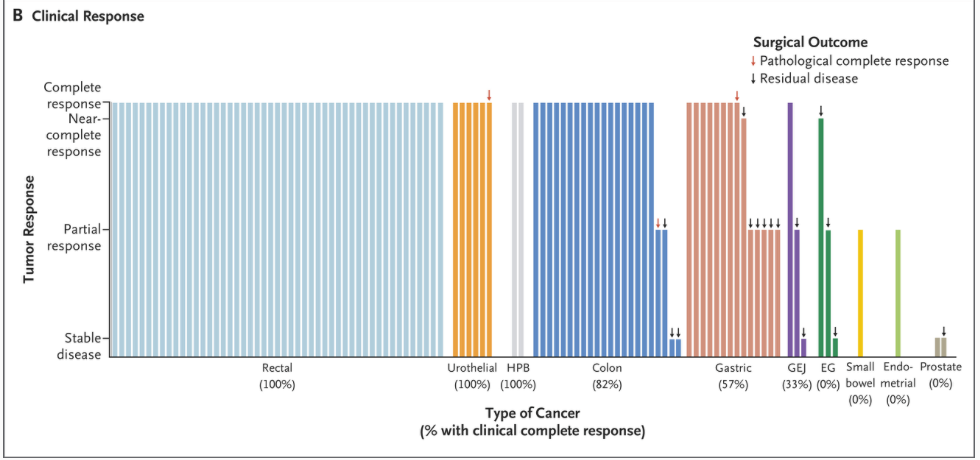
- Cohort 1 (Rectal cancer): 100% (49/49) achieved CCR; all opted for nonoperative management.
- Cohort 2 (Nonrectal solid tumors): 65% (35/54) achieved CCR; 61% (33/54) chose nonoperative management.
Across both cohorts, CCR was seen in 82% (84/103) of patients who completed treatment; 80% (82/103) proceeded without surgery.
In Cohort 1, sustained clinical complete response (CCR) at 12 months was observed in 76% of patients (37 out of 49), successfully meeting the primary efficacy endpoint. Overall, the 2-year recurrence-free survival rate across both cohorts was 92% (95% CI, 86-99). Specifically, Cohort 1 demonstrated an impressive recurrence-free survival rate of 96% (95% CI, 90-100), while Cohort 2 had a slightly lower rate at 85% (95% CI, 70-100). Notably, disease recurrence occurred in only five patients, with four cases restricted to the lymph nodes, all of which were effectively managed through either resumed PD-1 blockade therapy or surgical intervention.
Safety Profile
Serious adverse events (grade 3 or higher) were rare, occurring in approximately 5% of patients, including single cases each of diabetes, lung infection, hypothyroidism, encephalitis, neutropenia, and febrile neutropenia.
- 65% experienced adverse events, predominantly mild (grade 1 or 2).
- Common mild adverse effects: Fatigue (23%), rash/dermatitis (21%), pruritus (19%).
Response Assessment Metrics
- Median time to complete response on imaging or endoscopy: 6.2 months
- Median time to negative biopsy: 1.5 months
- Median time to clearance of circulating tumor DNA: 1.4 months
- High concordance (89.5%) between circulating tumor DNA and biopsy results
ctDNA showed high concordance with biopsy results (Kappa=0.76), proving reliable as a non-invasive monitoring tool.
Genomic Analyses
- High genomic relatedness (99.6%) between baseline and post-treatment tumors, with minimal variation in tumor mutational burden and microsatellite instability scores.
- Only two instances indicated new primary tumors rather than recurrence, demonstrating stability of genomic signatures post-treatment.
Clinical Implications
This study provides compelling evidence that neoadjuvant PD-1 blockade therapy offers a viable nonoperative management strategy for early-stage dMMR solid tumors across multiple cancer types. The approach yielded particularly impressive results for rectal cancer (100% complete response rate) and demonstrated efficacy in various nonrectal tumors.
Importantly, the option for curative resection was not compromised in any patient, despite the extended 6-month treatment duration. Three rectal cancer patients were subsequently able to conceive and deliver healthy children—an outcome not possible with standard treatment.
Discussion and Key Takeaways
- Nonoperative management could become standard for early-stage dMMR cancers, pending validation from larger, possibly randomized, studies.
- Further investigation into optimizing treatment duration and combination therapy may enhance complete response rates in tumor types showing lower response.
- Circulating tumor DNA emerged as a promising biomarker for monitoring therapeutic response in real-time, with over 90% of early-stage tumors detected at baseline and high concordance with biopsy results.
- ctDNA as a routine monitoring tool could become pivotal, significantly improving real-time assessment of treatment effectiveness and recurrence detection.
Neoadjuvant PD-1 blockade using dostarlimab leads to significant organ preservation and nonoperative management feasibility in patients with early-stage mismatch repair–deficient tumors. These compelling results highlight the potential for broader application across multiple solid tumor types, significantly reshaping future cancer treatment paradigms.
You can read the full article in NEJM

You can Read Immunotherapy for Colon Cancer Article on OncoDaily