The primary analysis of the Phase 3 DESTINY-Gastric04 trial was presented by Dr. Kohei Shitara at the 2025 ASCO Annual Meeting. The study compared trastuzumab deruxtecan (T-DXd) to the standard second-line regimen of ramucirumab (RAM) plus paclitaxel (PTX) in patients with HER2-positive unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma (GC/GEJA).
What Is the DESTINY-Gastric04?
DESTINY-Gastric04 was a global, open-label, Phase 3 clinical trial that enrolled 494 patients with HER2-positive advanced gastric or GEJ cancer. Patients had to have confirmed HER2 positivity, defined as IHC 3+ or IHC 2+ with ISH positivity, and prior progression on a trastuzumab-containing regimen. They were randomized 1:1 to receive either T-DXd at 6.4 mg/kg every three weeks (n=246) or the standard combination of ramucirumab plus paclitaxel (n=248). The primary endpoint of the study was overall survival (OS), with secondary endpoints including progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and safety.
DESTINY-Gastric04 Design and Patient Population
At the time of data cutoff (October 24, 2024), 246 patients had received T-DXd and 248 had received RAM + PTX. The median duration of treatment was 5.4 months (range, 0.7–30.3) for T-DXd and 4.6 months (range, 0.9–34.9) for RAM + PTX. The median follow-up for OS was 16.8 months in the T-DXd arm and 14.4 months in the RAM + PTX arm.
Efficacy Results of DESTINY-Gastric04
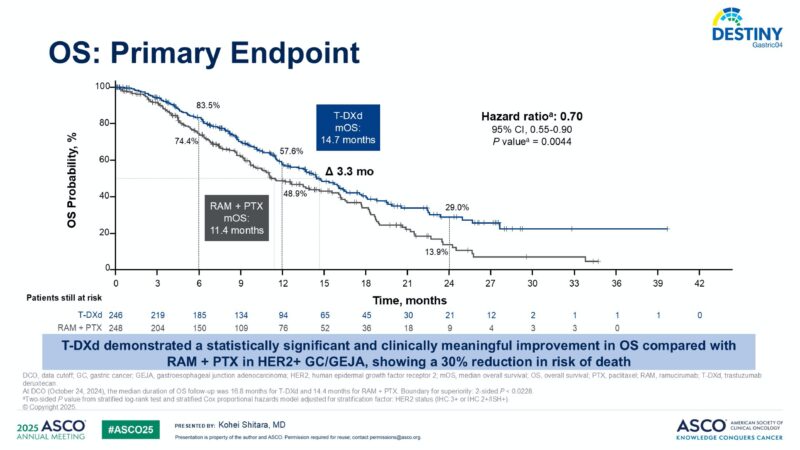
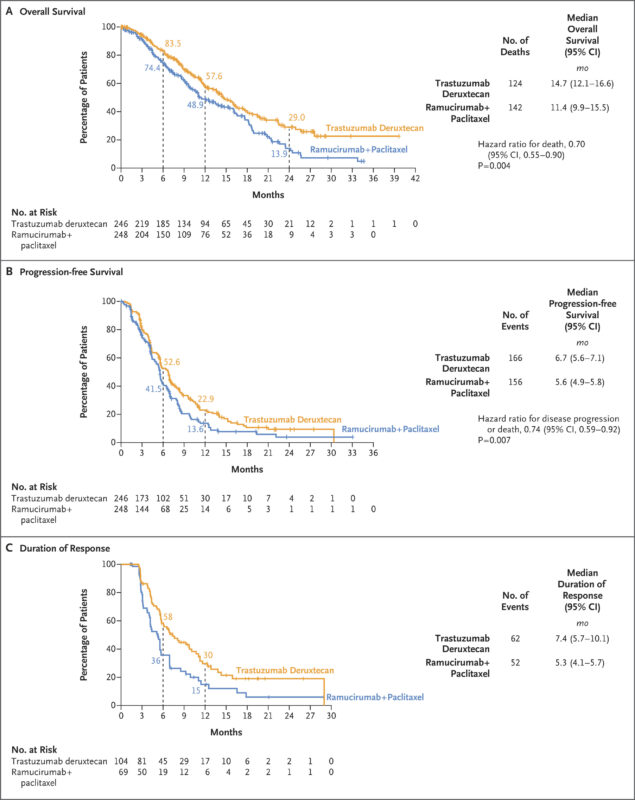
T-DXd demonstrated a statistically significant and clinically meaningful improvement in OS compared with RAM + PTX. Median overall survival was 14.7 months (95% CI: 12.1–16.6) with T-DXd versus 11.4 months (95% CI: 9.9–15.5) with RAM + PTX. This translated to a 30% reduction in the risk of death (hazard ratio [HR], 0.70; 95% CI: 0.55–0.90; P = 0.0044).
Progression-free survival was also significantly improved: median PFS was 6.7 months (95% CI: 5.6–7.1) for T-DXd compared to 5.6 months (95% CI: 4.9–5.8) for RAM + PTX (HR, 0.74; 95% CI: 0.59–0.92; P = 0.0074).
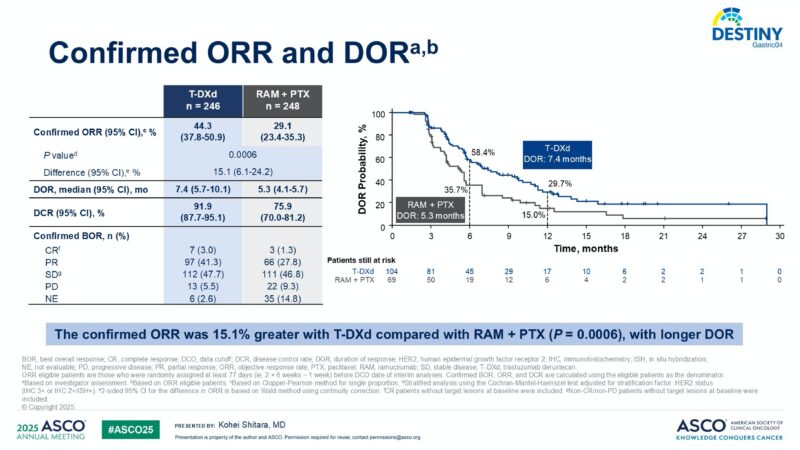
Confirmed objective response rate was 44.3% (95% CI: 37.8–50.9) with T-DXd versus 29.1% (95% CI: 23.4–35.3) with RAM + PTX (P = 0.0006). Disease control rate was notably higher with T-DXd at 91.9% (95% CI: 87.7–95.1) versus 75.9% (95% CI: 70.0–81.2) for the comparator arm.
Safety and Tolerability
Treatment-emergent adverse events (TEAEs) occurred in nearly all patients: 100% in the T-DXd arm and 97.9% in the RAM + PTX arm. Grade ≥3 TEAEs were reported in 68.0% of patients receiving T-DXd versus 73.8% with RAM + PTX.
Serious adverse events occurred in 41.0% of T-DXd patients compared to 43.3% with RAM + PTX. Treatment discontinuation due to AEs occurred in 14.3% of patients in the T-DXd arm and 17.2% in the RAM + PTX arm.
Of special interest was drug-related interstitial lung disease (ILD) or pneumonitis, which was reported in 13.9% of patients treated with T-DXd (n=34). Most events were low grade, with only one Grade 3 ILD case and no Grade 4 or 5 cases. In contrast, ILD/pneumonitis occurred in 1.3% (n=3) of patients in the RAM + PTX group, including two Grade 3 and one Grade 5 event.
Key Takeaways
- T-DXd significantly prolonged overall survival compared to RAM + PTX in HER2-positive advanced GC/GEJA patients in the second-line setting.
- Progression-free survival and response rates were also notably improved with T-DXd.
- The safety profile of T-DXd was consistent with prior studies, with no new safety signals, although ILD remains a key adverse event to monitor.
- These findings reinforce trastuzumab deruxtecan as a new second-line standard of care for HER2-positive advanced gastric and gastroesophageal cancers.
The DESTINY-Gastric04 trial represents a major step forward in the treatment landscape for HER2-positive gastric cancer, offering patients a more effective and targeted therapy following first-line trastuzumab failure. This results were also published in NEJM May 31, 2025.
Read Full Abstract on ASCO Official Website.
What People Are Saying About the DESTINY-Gastric04 Trial?
Dr. Kohei Shitara shared a heartfelt message on his X page following the presentation of the DESTINY-Gastric04 trial at ASCO 2025 and its simultaneous publication in The New England Journal of Medicine. He wrote:
“A tribute to Dr. Agatsuma was a meaningful moment during my DESTINY-Gastric04 presentation at #ASCO25 with @NEJM publication. I deeply respect him and the entire team behind the development of T-DXd.”

Dr. Amol Akhade, a medical oncologist and hemato-oncologist based in India, posted on his X page about the DESTINY-Gastric04 trial results presented at ASCO 2025. He highlighted that trastuzumab deruxtecan (T-DXd) significantly improved overall survival over ramucirumab plus paclitaxel in second-line HER2-positive gastric/GEJ cancer.
“New Standard in 2L HER2+ Gastric Cancer?
DESTINY-Gastric04 (NEJM) T-DXd vs Ramucirumab+Paclitaxel
N=494, post-trastuzumab HER2+ GEJ/Gastric AC
Primary OS benefit: 14.7 mo vs 11.4 mo, HR 0.70 | p=0.004
PFS: 6.7 vs 5.6 mo
ORR: 44.3% vs 29.1%
But… some concerns
ILD/pneumonitis in 13.9%
(though mostly grade 1–2)
No in-trial crossover, yet ~26% in control arm got T-DXd post-progression
HER2 heterogeneity not fully addressed
Subgroup CI >1.0 in many cohorts
Open-label + regional variations = interpret cautiously
Still, this cements T-DXd as new 2L SOC if HER2 remains positive post 1L.
HER2 “reconfirmation” post-trastuzumab is now non-negotiable.”
More posts featuring ASCO25.


