At the ESMO Congress 2025 in Berlin, Dr. Kanwal Raghav (Houston, USA) presented the final analysis of the phase II DESTINY-CRC02 trial (NCT04744831), a multicenter study evaluating trastuzumab deruxtecan (T-DXd) in HER2-positive metastatic colorectal cancer (mCRC). With extended follow-up, the data confirm durable activity of T-DXd 5.4 mg/kg and reaffirm this lower dose as the optimal regimen balancing efficacy and safety in a difficult-to-treat population.
Background
HER2 amplification defines a distinct molecular subtype of colorectal cancer, representing approximately 2–3% of metastatic cases. Although HER2-targeted combinations such as trastuzumab + tucatinib or trastuzumab + pertuzumab have demonstrated meaningful responses in refractory settings, outcomes remain inferior to those achieved in other HER2-driven malignancies.
Trastuzumab deruxtecan (T-DXd), an antibody-drug conjugate combining anti-HER2 targeting with a topoisomerase I inhibitor payload, has shown robust efficacy in HER2-positive breast and gastric cancers. Its potential in mCRC was first signaled by the DESTINY-CRC01 trial, which reported a 45% objective response rate. The DESTINY-CRC02 study further refined the dosing strategy by directly comparing 5.4 mg/kg and 6.4 mg/kg every 3 weeks, establishing a new benchmark for HER2-targeted therapy in mCRC.
Study Design
DESTINY-CRC02 enrolled 122 patients with HER2-positive (IHC 3+ or IHC 2+/ISH+) mCRC, irrespective of RAS status. All participants had progressed after prior systemic therapy, including fluoropyrimidines, oxaliplatin, and irinotecan.
- Stage 1: 40 patients each received T-DXd 5.4 mg/kg or 6.4 mg/kg every 3 weeks.
- Stage 2: An additional 42 patients were treated at 5.4 mg/kg, confirming efficacy at this dose.
The primary endpoint was confirmed objective response rate (cORR) by blinded independent central review. Secondary endpoints included duration of response (DOR), overall survival (OS), and safety—particularly treatment-emergent adverse events (TEAEs) and interstitial lung disease (ILD).

Key Findings
At the first interim analysis (Data Cutoff Nov 1 2022), median follow-up was 8.9 months for 5.4 mg/kg and 10.3 months for 6.4 mg/kg.
- Confirmed ORR: 37.8 % (95 % CI 27.3–49.2) for 5.4 mg/kg vs 27.5 % (14.6–43.9) for 6.4 mg/kg.
- Median DOR: 5.5 months (4.2–8.1) vs 5.5 months (3.7–NE).
- Median OS: 13.4 months (12.5–16.8) for 5.4 mg/kg and not evaluable (9.9–NE) for 6.4 mg/kg.
At this stage, T-DXd showed promising activity with manageable toxicity and suggested that the lower dose might offer comparable efficacy with reduced adverse events.
Final Results
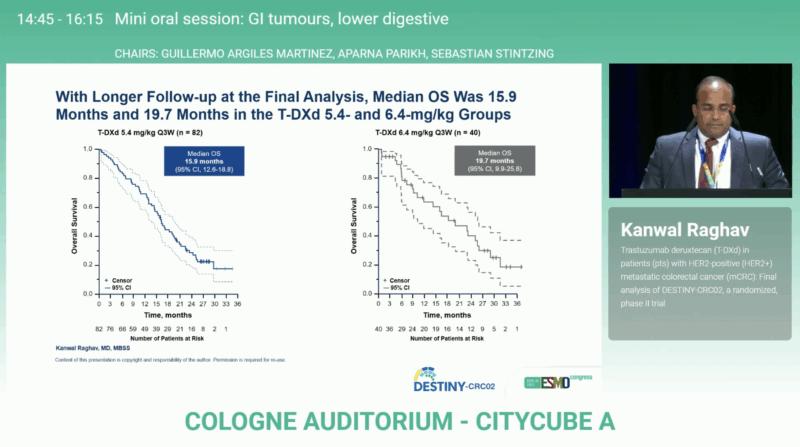
At the final data cutoff (December 4, 2024), median follow-up had increased to 14.2 months for the 5.4 mg/kg group and 12.7 months for the 6.4 mg/kg group—representing an extension of 5.3 and 2.4 months, respectively, over the primary analysis.
Efficacy outcomes remained stable
- Confirmed ORR: 37.8% (95% CI 27.3–49.2) for 5.4 mg/kg vs 27.5% (14.6–43.9) for 6.4 mg/kg
- Median DOR: 5.5 months in both arms
- Median OS: 15.9 months (12.6–18.8) at 5.4 mg/kg and 19.7 months (9.9–25.8) at 6.4 mg/kg
- Median PFS: 5.8 months (4.6–7.0) at 5.4 mg/kg and 5.5 months (4.2–7.0) at 6.4 mg/kg.
These results confirm that T-DXd induces clinically meaningful and durable responses across doses, consistent with the earlier analysis. The slightly longer median OS observed in the higher-dose arm did not offset its increased toxicity burden.
Safety Profile
Safety data mirrored earlier findings, with no new signals emerging after extended observation.
- Grade ≥ 3 drug-related TEAEs: 42.2% at 5.4 mg/kg vs 48.7% at 6.4 mg/kg
- Adjudicated drug-related ILD: 9.6% (2 grade 1; 6 grade 2) at 5.4 mg/kg vs 17.9% at 6.4 mg/kg
- Drug-related deaths: 1 (1.2%) in the 5.4-mg/kg arm and 1 (2.6%) in the 6.4-mg/kg arm
The incidence of ILD—a class-defining adverse event of T-DXd—was dose-dependent and clinically significant. While mostly low-grade and manageable, the persistence of rare grade 5 cases reinforces the need for vigilant monitoring and early corticosteroid intervention.
Clinical Interpretation
The consistency of efficacy across analyses and the manageable toxicity profile strongly support T-DXd 5.4 mg/kg Q3W as the preferred dose for HER2-positive mCRC. Its cORR of ~38%, median OS of ~16 months, and DOR beyond 5 months represent notable progress in a heavily pretreated setting where standard chemotherapy typically achieves <5% response rates.
Importantly, activity was observed irrespective of RAS status, suggesting that HER2 amplification may serve as a dominant therapeutic driver even in RAS-mutant tumors—a finding that could broaden the eligible population in future studies.
The results align with the paradigm shift seen in other solid tumors: antibody–drug conjugates are redefining therapeutic sequencing and enabling precision targeting even in historically resistant disease types.
You can read the full abstract here.
Conclusion
The DESTINY-CRC02 final analysis reaffirms trastuzumab deruxtecan 5.4 mg/kg as the preferred regimen for HER2-positive mCRC, combining robust and durable responses with a manageable safety profile. With a confirmed ORR of 38% and median OS near 16 months, T-DXd delivers meaningful clinical benefit for patients who have exhausted standard therapies. No new safety concerns emerged, and ILD remained within an expected, monitorable range.
These results consolidate T-DXd as a key targeted therapy for HER2-positive mCRC and strengthen the foundation for future studies exploring its role in earlier treatment lines and in rational combinations.
Read more about PEGASUS Trial at ESMO 2025: Liquid Biopsy-Guided Adjuvant Therapy in Resected Colon Cancer on OncpDaily.


