Cervical cancer is the fourth leading cancer diagnosis in women worldwide, with around 660,000 new cases and 350,000 deaths in 2022. In the U.S., an estimated 13,360 new cases and 4,320 deaths are expected in 2025. World Health Organization, WHO website, 2024. American Cancer Society, ACS website, 2025. Cervical cancer often affects women in early to mid-adulthood and causes financial hardship, stigma, and issues like sexual dysfunction or infertility, especially with late diagnosis and limited care access. H Endale, Cancer Manag Res, 2022. Early detection significantly improves survival, with over 90% five-year survival for early-stage cases, compared to 20% for advanced cases. Centers for Disease Control and Prevention, CDC website, 2024.
Screening programs, including Pap smears and HPV testing, reduce incidence and mortality where widely implemented, also lowering healthcare costs and enabling less aggressive treatments. American Cancer Society, ACS website, 2025. S Basoya, Cureus, 2022.

Photo: Depositphotos
This article is a basic guide to cervical cancer screening, explaining the role of the Pap test and HPV testing in detecting cellular changes that could lead to cervical cancer. It also covers who should get screened, how often, and why these tests are key to prevention and early diagnosis.
What Is Cervical Cancer, And Why Is Cervical Cancer Screening Important?
Cervical cancer usually begins with precancerous changes in the cervix, most often caused by persistent infection with high-risk human papillomavirus (HPV). Smoking, immune suppression, reproductive history, socioeconomic factors, and not being vaccinated for HPV also play significant roles. The disease typically develops slowly and can often be detected early through screening. Symptoms often don’t appear in early stages; later signs include abnormal bleeding, pelvic pain, and unusual discharge. National Cancer Institute, NIH website, 2023, 2024.
Regular cervical cancer screening leads to earlier detection. Women screened at least every 5.5 years have a 92% lower risk of cervical cancer mortality. Screening reduces the occurrence of stage 1A cancers by 67% and advanced-stage cancers by 95%. When detected early at a localized stage, cervical cancer five-year survival rates exceed 90%. Worldwide, observational studies indicate a mortality reduction ranging from 41% to over 90%, which aligns with the WHO’s estimate that screening can decrease deaths by approximately 80% or more. R Landy, Br J Cancer, 2016. L Bruni, Lancet Glob Health, 2022.
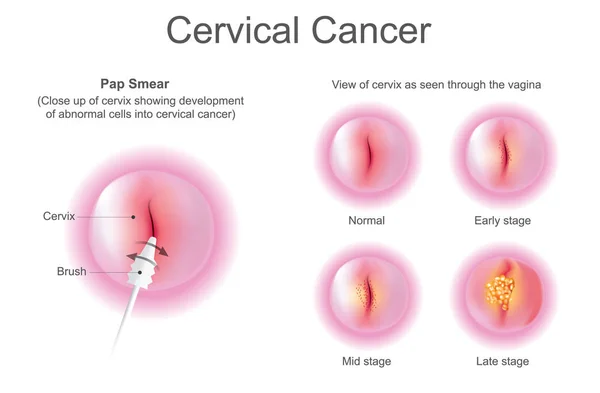
Photo: Depositphotos
Pap Smear: The Traditional Screening Test
A Pap test, also known as a Pap smear or cervical cytology, is a screening method used to collect cells from the cervix for examination under a microscope. Its main purpose is to detect precancerous or cancerous changes in cervical walls, allowing for early detection of cervical cancer, when it is most treatable. Beyond cancer screening, it can also reveal infections and inflammation. Mayo Clinic website, 2024. Canadian Cancer Society, CCS website, 2025.
During a Pap test, a speculum is used to view the cervix, and a sample of cervical cells is collected with a small brush or spatula, which is then placed into a special solution or onto a glass slide and sent to a laboratory to be examined for abnormalities. The procedure usually takes a few minutes to complete and may cause mild discomfort, but generally does not cause pain. M Kamal, Cytojournal, 2022.
HPV Testing: Detecting the Virus Behind Cervical Cancer
HPV testing screens for high-risk human papillomavirus types that are responsible for most cervical cancers, particularly HPV 16 and 18. The test checks cervical cell samples for HPV DNA or RNA, detecting infections that could develop into cancer. While it does not diagnose cancer directly, it helps identify those at increased risk, guiding timely follow-up examination. Often used alongside or instead of Pap smears, HPV testing offers a more sensitive way to detect potential issues early, leading to better prevention. American Cancer Society, ACS website, 2025.
Co-Testing: Dual Approach for Enhanced Detection
Pap and HPV co-testing involves the same sampling technique as the individual tests and uses one sample to run both tests simultaneously, analyzing it for both cytologic abnormalities and high-risk HPV types. This approach combines the benefits of each test, enhancing the detection of high-grade lesions and invasive cancers while lowering false-negative rates and allowing for longer screening intervals – five years if both results are negative. Unlike HPV testing alone, it does not increase unnecessary colposcopy rates.
Co-testing is more effective than both Pap smear and HPV testing when used individually. It has significantly reduced cervical cancer incidence and mortality in regions where it is routinely used and is a preferred option when primary HPV testing is not available. National Cancer Institute, NCI Dictionaries. NCI website, 2025. J Cuzick, Gynecol Oncol, 2021.
Determining the Right Time for Cervical Screening
The United States Preventive Services Task Force (USPSTF) recommends screening for cervical cancer every 3 years with cervical cytology (Pap smear) alone in women aged 21 to 29 years. For women aged 30 to 5 years, the USPSTF recommends screening every 3 years with cervical cytology alone, every 5 years with HPV testing alone, or every 5 years with co-testing.
In 2020, the American Cancer Society updated its cervical cancer screening guidelines, recommending that screening begin at age 25, rather than age 21 as previously advised. The preferred method is primary HPV testing (an HPV test done individually) every five years through age 65. If primary HPV testing is not available, screening may be done with either co-testing every five years or a Pap test alone every three years.
Many European countries have transitioned to or are in the process of transitioning to primary HPV testing as their primary screening method. Some programs incorporate self-sampling for HPV testing to increase uptake. Screening intervals are typically every 5 years, and the starting and stopping ages are generally around 25-30 years to 65 years, with updates planned to consider vaccinated populations. European Cancer Organisation, European Cancer Organisation Website, 2021. International Agency for Research on Cancer, WHO website, 2025.
Screening may be discontinued after the age of 65 for those who have had regular screening, with normal results in the previous 10 years and no history of serious cervical precancer (CIN2 or higher) in the past 25 years. It is important to note that under current guidelines, individuals vaccinated against human papillomavirus (HPV) are advised to follow the same screening protocols as those who are unvaccinated. National Cancer Institute, NCI website, 2020.
The American Cancer Society emphasizes that the most important aspect of cervical cancer screening is regular screening, regardless of the test used.
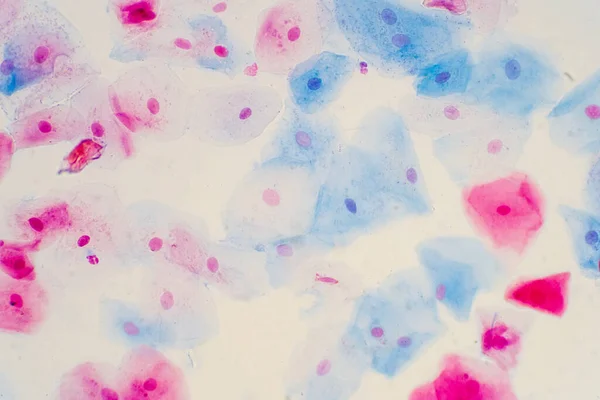
Flat epithelial cells of the human cervix under a microscope. Photo:Depositphotos
Special Considerations for Those with Hysterectomy
Cervical cancer screening recommendations differ based on the type of hysterectomy performed and a woman’s age.
Individuals who have had a total hysterectomy (where both the uterus and the cervix are removed) for benign reasons, such as fibroids or prolapse, and have no history of cervical pathology at the time of surgery, do not require further cervical cancer screening. The risk of developing vaginal cancer is extremely low, and routine Pap or HPV testing of the vaginal vault is not recommended in these case. American Cancer Society, ACS website, 2021.
If there is a history of high-graded cervical intraepithelial neoplasia (CIN2+), adenocarcinoma in situ (AIS), or cervical cancer, the screenings should be continued after hysterectomy. Recommended follow-up involves annual co-testing of the vaginal vault (Test of Cure, ToC) until two consecutive negative results are achieved (HPV is not detected and cytology is normal), after which further testing may be discontinued. If the ToC was completed before hysterectomy and the hysterectomy specimen is clear of disease, no additional screening is needed. If the ToC was not completed before hysterectomy, it should be completed after surgery. American Cancer Society, ACS website, 2021.
If histology at the time of hysterectomy is not known, one baseline cytology (Pap test) of the vaginal vault is recommended. If the results are normal, no further screening is required. If the results are positive, follow-up with colposcopy and HPV testing is needed. Cancer Council Australia, Cervical Cancer Screening Clinical Management Guidelines, 2025.
Photo: Depositphotos
Individuals who have undergone a subtotal hysterectomy, where the cervix is left intact, should continue with routine cervical screening, including HPV testing every 5 years or as recommended by national guidelines. American Cancer Society, ACS website, 2021. Cancer Council Australia, Cervical Cancer Screening Clinical Management Guidelines, 2025.
If there is a history of VAIN (vaginal intraepithelial neoplasia), gynecologic cancer, or incomplete excision of high-grade lesions (CIN2/3), follow-up should be individualized by a specialist. Persistent HPV infection after hysterectomy is also a key factor calling for careful monitoring – especially for those previously treated for CIN – given the increased risk of vaginal neoplasia. X Liu, Pak J Med Sci, 2013.
How Should I Prepare for a Cervical Screening Test?
To help ensure the most accurate results, it is best to avoid scheduling a test during the menstrual period and to refrain from sexual intercourse, douching, or using vaginal products for at least two days before the appointment.
The procedure begins with a pelvic exam, during which the healthcare provider inserts a speculum into the vagina, which holds the vaginal walls apart, allowing a clear view of the cervix. Once the cervix is visible, a soft brush and a flat scraping tool – a spatula – are used to collect cells from the cervix. The brush is used to sample the inner canal (endocervix), while the spatula collects cells from the outer surface – the ectocervix. The collected cells are then preserved by placing them in a liquid solution or smearing them onto a glass slide and sent for a lab analysis.
The entire procedure takes a few minutes, is typically mildly uncomfortable but not painful, and individuals can resume their normal daily activities immediately after the test. Mild cramping or light spotting may occur after the test, but it usually resolves shortly afterwards. M Kamal, Cytojournal, 2022. Mayo Clinic, Mayo Clinic website, 2024.
How Effective Is Self-Collection Compared to Clinic Testing?
Self-collection is a safe and effective cervical screening method that allows individuals to collect their vaginal sample for HPV testing. Research shows that self-collected samples are just as accurate as clinician-collected ones in detecting high-risk HPV.
This method is a reliable option for people aged 25 to 74 who are due or overdue for cervical screening. A healthcare provider supplies the kit – a brush or a swab and a test tube, and provides instructions; the sample is collected by inserting the swab a few centimetres into the vagina and rotating for 20-30 seconds to collect cells. After collection, the swab is returned for lab testing. While self-collection only tests for HPV and not cellular abnormalities, any positive result will prompt follow-up with clinician-based testing or colposcopy.
Self-collection is now available in many clinics, increasing access to cervical screening. It offers a more private and comfortable alternative to the traditional speculum exam. Offered alongside traditional clinician collection, it allows individuals to choose the approach that best suits them. S Reynolds, National Cancer Institute Website, 2024.
Normal, Abnormal, Unsure: Understanding Results
For the Pap test results – whether normal or abnormal – to be valid, the sample collected must be of sufficient quality and quantity for accurate analysis. If the sample is inadequate for evaluation, the result is reported as “unsatisfactory”, and the test should be repeated in 2-4 months.
A normal Pap test result (negative result, negative for intraepithelial lesion or malignancy) means that no abnormal cervical cells were found in the sample collected from the cervix, and there is no evidence of precancerous or cancerous changes. Itt does not guarantee that the individual is free from all sexually transmitted diseases or other health conditions. Routine screening should continue as recommended, since Pap tests are not 100% sensitive, and cervical changes can develop over time.
An abnormal Pap test result (positive result) indicates the presence of cellular changes in the sample collected from the cervix. This, however, does not necessarily suggest the presence of cervical cancer. In many cases, the changes are related to infections such as HPV, inflammation, or mild abnormalities that usually resolve on their own. Abnormal results are classified reflecting the degree of cellular abnormality and associated risk.
The most common finding is atypical squamous cells of undetermined significance (ASC-US), which indicates minor cellular changes, though it is unclear whether the changes are caused by HPV infection or other factors such as irritation, yeast infection, polyps in the uterus or hormonal fluctuations. An HPV test is often done to check if the changes are linked to an HPV infection. If the result is negative, estrogen cream may be used to address possible hormone-related causes. A positive result requires additional follow-up.
Low-grade squamous intraepithelial lesion (LSIL) refers to mild abnormalities, usually caused by HPV. This might also be called mild dysplasia or cervical intraepithelial neoplasia grade 1 (CIN1).
Atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesions (ASC-H): this result indicates the presence of abnormal squamous cells that may represent a high-grade lesion (HSIL), but the findings are not definitive.
High-grade squamous intraepithelial lesions (HSIL) refer to significant cellular changes with a higher risk of progressing into cervical cancer if left untreated. This might also be called moderate to severe dysplasia or cervical intraepithelial neoplasia grade 2 or 3 (CIN2 and/or CIN3).
Atypical glandular cells (AGC) indicate abnormal changes in cells from the cervix or endometrium that may be associated with a wide range of conditions, from benign causes such as polyps or endometriosis to high-risk lesions or malignancy. The risk is higher if the AGC is positive for high-risk HPV types.
Adenocarcinoma in situ (AIS) is a precancerous lesion found in the glandular tissue of the cervix, with the potential to progress to cervical adenocarcinoma.
Cervical cancer cells – either squamous cell carcinoma or adenocarcinoma – are rarely detected in those screening regularly. A biopsy is needed to confirm the diagnosis. National Cancer Institute, NIH website, 2024. American Cancer Society, ACS website, 2025.
For HPV testing, a negative result means there was no high-risk HPV found, indicating a low risk of developing cell changes; routine screening should continue as recommended. A positive result indicates the presence of high-risk HPV but not necessarily cancer – if HPV is present without cellular abnormalities, it usually resolves on its own. An unsatisfactory result means the sample couldn’t be properly analyzed. National Cancer Institute, NIH website, 2024.
What’s Next? Follow-up After Abnormal Results
After an abnormal Pap test, follow-up depends on the severity of the abnormality and the patient’s history. High-grade results or persistent HPV infections typically require colposcopy and biopsy to confirm the diagnosis and guide treatment. Lower-grade changes may require repeat Pap or HPV testing at intervals determined by risk.
Follow-up plans are personalized considering current and past test results, treatments, and individual health factors. For treated high-grade lesions, ongoing HPV testing every 3 years is recommended due to the long-term risk of recurrence. After cervical cancer treatment, follow-up visits are more frequent in the first few years and become less so over time. It is important to report any new symptoms between visits to ensure early detection and timely care.
The first step after a positive HPV result is usually reflex cytology – a Pap test on the same sample to evaluate for cellular abnormalities. If HPV types 16 or 18 are detected, or if cytology reveals high-grade changes, immediate colposcopy is recommended. For other high-risk HPV types with normal or low-grade cytology, repeat testing at 12 and 24 months is advised, with colposcopy if the virus persists. If the sample was self-collected, a follow-up provider-collected sample is needed to confirm results.
Timely follow-up after an abnormal Pap test is vital for early detection and prevention of cervical cancer. It allows for personalized care based on current and past results, ensuring appropriate next steps — repeat testing, colposcopy, or treatment. Regular monitoring is especially important after treatment to detect recurrence early, particularly within the first few years. Follow-up also helps identify new symptoms promptly. Skipping or delaying follow-up increases the risk of undetected progression and reduces treatment success. National Cancer Institute, NIH website, 2024. American College of Obstetricians and Gynecologists, ACOG guidelines, 2020.
From Pap Smears to AI: Progress And Limits in Cervical Cancer Screening
While cancer screening is essential for early detection and prevention, it comes with certain limitations. Not all cancers are prevented or detected through screening – some may be missed due to false negative results or rapidly progressing disease. False-positive tests can lead to unnecessary follow-up and anxiety or overtreatment. In many low-resource settings, limited services, shortage of trained staff, and insufficient funding restrict access to effective screening programs. Current guidelines on when to stop screening often depend on having decades of prior results, which may not be available, making follow-up decisions difficult. Pap smears require expert interpretation and have variable sensitivity, and visual inspection methods, often used in resource-limited settings, have lower specificity, leading to higher risk of overtreatment.
Lastly, the effectiveness of screening relies heavily on patient participation and adherence to follow-up care, which can be affected by various social, cultural, and logistical challenges. SCCPS, Cervical Screening Clinical Management Guidelines, 2025. R B Perkins, JAMA Netw Open, 2025.
Modern cervical cancer screening has become more effective and accessible. Primary HPV testing, now increasingly adopted, offers greater sensitivity than Pap smears and allows for longer intervals between screenings. Self-collection kits, now FDA-approved, make participation easier and more comfortable. Risk-based guidelines help guide individualized follow-up and intervals, based on HPV status, cytology, and patient history, helping balance the benefits and potential harms. The increasing impact of HPV vaccination is starting to change screening needs. Finally, advances in laboratory technology and digital tools, including the integration of artificial intelligence, are enhancing the speed and accuracy of diagnosis. NIH, National Cancer Institute website, 2025. M Rayner, Healthcare (Basel), 2023. L Pantanowitz, Cancer Cytopathol, 2022.
You Can Also Read How Artificial Intelligence Is Transforming Cancer Care in 2025: Diagnosis, Treatment, Clinical Trials, and Screening by Oncodaily
So, Why Risk It?
Today’s technology helps detect subtle changes before they become serious, and while no test is perfect – there is always a risk of something being missed or misinterpreted – getting screened is the simplest step one can take to take care of their long-term well-being. Testing takes a few minutes, but it can make a long-lasting difference: it is surely an investment in safety and peace of mind. If there is uncertainty about which test is appropriate or where to start, talking to a healthcare professional can help clarify the options and choose a suitable screening plan.
You Can Also Read USA vs Europe: Who Does Cancer Screening Better? by Oncodaily
Written by Seda Adibekyan
FAQ
What causes cervical cancer and what are the risk factors?
Cervical cancer is primarily caused by persistent infection with high-risk types of human papillomavirus (HPV), especially types 16 and 18, which cause about 70% of cases. Other risk factors include smoking, HIV infection, having multiple sexual partners, early sexual activity, long-term use of birth control pills, having three or more children, a weakened immune system, and family history. Women living with HIV are about six times more likely to develop cervical cancer.
What are the symptoms of cervical cancer?
Early-stage cervical cancer often has no symptoms. When symptoms appear, they may include abnormal vaginal bleeding such as bleeding after intercourse, between periods, after menopause, or heavier and longer menstrual bleeding. Other symptoms include increased vaginal discharge, pain during sexual intercourse, and unexplained pelvic or back pain.
How can cervical cancer be prevented and detected early?
Cervical cancer can be effectively prevented through HPV vaccination and regular screening tests such as Pap smears and HPV tests. Screening identifies precancerous lesions that can be treated before they develop into cancer. Early detection greatly improves treatment success and survival rates. Women living with HIV and those at higher risk should be screened more frequently. The World Health Organization and health authorities recommend HPV vaccination for adolescents and routine cervical cancer screening for women starting from age 25 or 30 depending on guidelines.
Are Pap smears painful?
Many believe Pap smears are painful, but the procedure is generally not painful, though it may cause slight discomfort or a minor pinch. The test involves inserting a speculum and collecting cervical cells with a soft brush, which most women tolerate well.
Do only sexually active women need Pap smears?
This is a common myth. Pap smears are recommended for all women starting at age 21 regardless of sexual activity because cervical cancer risk factors include more than just sexual transmission of HPV, such as genetics and immune status.
If I have the HPV vaccine, do I still need Pap smears?
Yes. The HPV vaccine protects against certain high-risk HPV strains but not all. Regular Pap smears remain essential for early detection of any abnormal cervical cells that could lead to cancer.
If I have only one sexual partner or am in a long-term monogamous relationship, do I still need Pap tests?
Yes. HPV can lie dormant for years and may not show up immediately on tests. Even with one partner, persistent HPV infection can occur, so regular screening is important to catch any changes early.
Does a Pap smear test for sexually transmitted infections (STIs)?
Pap smears primarily screen for cervical cell abnormalities and precancerous changes, not for STIs. While some STIs can increase cervical cancer risk, Pap tests are not designed to detect infections like chlamydia or gonorrhea.


