Cancer screening significantly reduces mortality by detecting cancer early, when treatment is more effective. According to the National Institutes of Health, between 1975 and 2020, nearly 4.75 million deaths were prevented through screening. ESMO highlights that it improves survival rates, reduces the need for invasive treatment, lowers costs, and helps prevent progression to advanced stages where treatment is less effective and options are limited.
The U.S. uses a decentralized approach to cancer screening, often depending on individual healthcare providers and patient decisions, while Europe targets 90% coverage through centralized, population-based screening programs. These differences reveal varying policy priorities in preventive healthcare delivery.
This article presents a data-driven comparison of cancer screening programs, uptake, and effectiveness in the United States and Europe, guided by the latest policy updates and clinical guidelines.
How Do Cancer Burdens Shape Screening Priorities in the USA and Europe?
According to ESMO, in 2025, the United States is projected to report approximately 2.04 million new cancer cases and 618,000 deaths, with the most common cancers being breast (in women), prostate (in men), lung, and colorectal. The leading causes of cancer death include lung, breast, prostate, colorectal, and pancreatic cancers. In the European Union, an estimated 3.2 million new cases and 1.28 million deaths are expected. (ESMO, ESMO Oncology News), 2025. Breast, colorectal, lung, and prostate cancers are the most frequently diagnosed, while lung, colorectal, breast, pancreatic, and prostate cancers are the top causes of mortality. C Santucci, Ann Oncol, 2025.
Breast, cervical, and colorectal cancers have well-established, organized population-based screening programs in most regions. F Vallone, Psychooncology, 2022. Prostate, lung, and gastric cancer screening are targeted to high-risk groups or high-incidence regions, with ongoing expansion and pilot programs, especially in Europe and parts of Asia. C Xia, Chin Med J, 2022. Screening program implementation and coverage vary by country, with ongoing efforts to expand access and reduce disparities.( European Commission, European Parliament website, 2022.)
Screening programs are often a response to where cancer burdens fall heaviest. This approach not only reduces mortality and gives patients a higher chance of successful treatment, but also lowers the overall cost of care by avoiding more complicated treatments later. It enables more effective, less aggressive treatment and supports better long-term outcomes for individuals and society.
Screening Program Structures: Organized vs Opportunistic
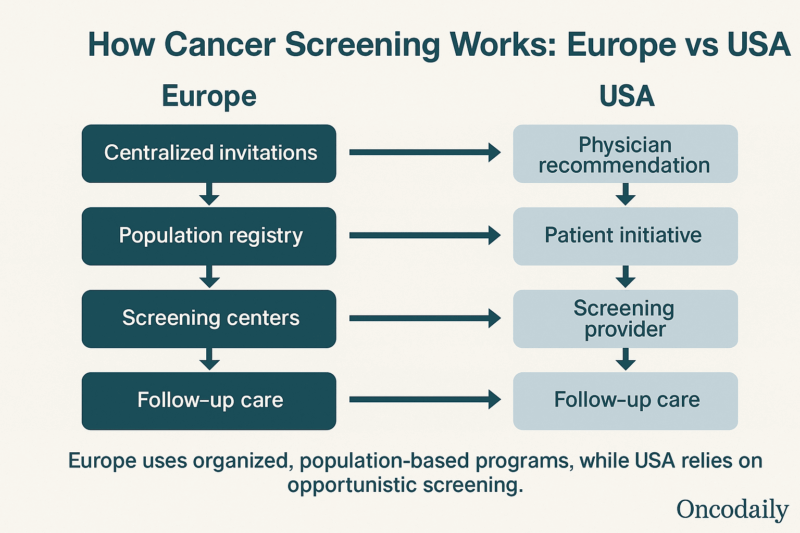
Europe’s approach to cancer screening is distinguished by its organized, population-based programs, which are underpinned by systematic invitations and robust quality assurance mechanisms.
Screening programs for breast, cervical, and colorectal cancer are systematically organized at the national or regional level. Eligible individuals within defined age groups are identified through population or health insurance registries, ensuring that invitations reach nearly all those at risk.European Commission, Report on the implementation of the Council Recommendation on cancer screening, 2021. Most European programs send personal invitations by mail to individuals in the target population. D B Vale, Eur J Cancer Prev, 2019.
The U.S. employs a non-population-based, decentralized system for cancer screening, characterized by reliance on physician recommendations, patient initiative, and variable adherence to guidelines. Unlike Europe’s organized programs, the U.S. lacks centralized, systematic invitations for screening. Participation depends on individual healthcare access and provider engagement. E B Peterson, Prev Med, 2017.
There is also complexity within the clinical guidelines: multiple organizations (United States Preventive Services Task Force, American Cancer Society, National Comprehensive Cancer Network) issue recommendations, leading to variability in screening protocols (e.g., differing start ages for mammography: USPSTF at 40, ACS at 45). Fragmented implementation, driven by differences in state policies, insurance coverage, and healthcare provider practices, contributes to disparities in access and outcomes. (President’s Cancer Panel, President’s Cancer Panel Report, 2022)
Structural differences between the U.S. and Europe shape cancer screening coverage. The U.S., despite lacking a publicly funded healthcare system for all, often reports higher screening rates due to opportunistic screening and broader age ranges. Europe uses organized, invitation-based programs aimed at equitable access, but coverage varies widely – ranging from 22% to 88% of U.S. levels depending on the country and cancer type. Eurostat, Eurostat Statistic Explained, 2024.
What Makes Cancer Screening So Effective in Finland, Denmark and the Netherlands?
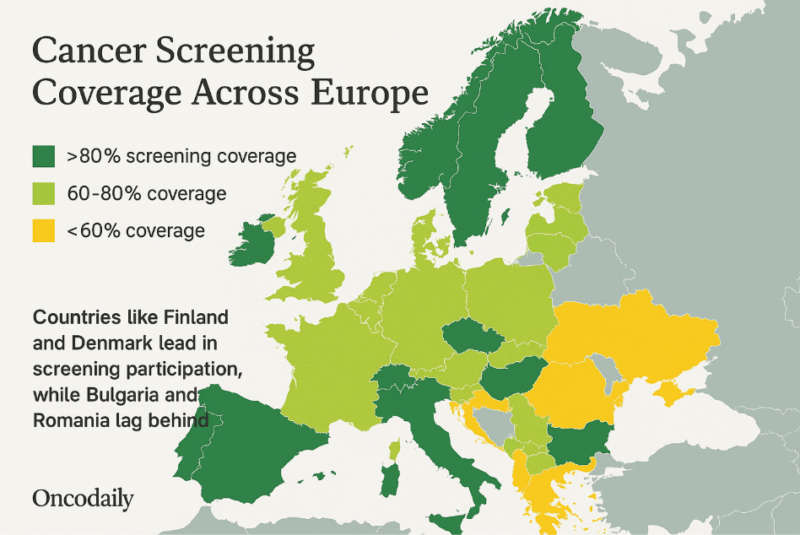
Several European countries have achieved high cancer screening uptake through organized, population-based programs. In Finland, over 80% of women aged 50-69 participated in breast cancer screening in 2022, with colorectal screening reaching 77.3% via FIT, among the highest in the EU. Cancer Country Profile 2025. Eurostat, Eurostat News, 2023. HPV-based cervical screening is also well-integrated into national programs. D Bhatia, Eurohealth, 2025.
The Netherlands reports a 68.4% colorectal screening rate, offers home-based HPV self-sampling, and maintains over 50% participation in breast cancer screening. (Eurostat, Statistics Explained 2024). Denmark achieved an 83% breast cancer screening rate in 2021 and maintains strong participation in colorectal and cervical screening through centralized invitations and quality tracking systems. OECD, Cancer Country Profile 2023, 2025. These successes reflect the benefits of organized, nationally coordinated programs supported by personalized invitations, robust research and monitoring, innovative delivery methods, strong public trust and high levels of literacy – all contributing to outcomes that closely align with EU quality standards. L Flander, Cancer Control, 2022.
Cancer Screening Approaches: Comparing Guidelines and Modalities in the USA and Europe
Cancer screening practices in the U.S. and Europe differ in terms of recommended age, screening intervals, and diagnostic methods.
Breast cancer screening in the U.S. typically begins at age 40, with the USPSTF recommending mammograms every two years up to age 74, while the ACS suggests annual screening from 45 to 54, then biennial after 55. MRI is also used for high-risk individuals. In Europe, most countries offer biennial mammography for women aged 50–69 through organized programs, with contrast-enhanced imaging under evaluation. M H Ebell, Public Health Rev, 2018.
For cervical cancer, the U.S. guidelines recommend screening women aged 21-65, using Pap smears and HPV co-testing every 3 to 5 years. Europe targets women aged 30–65 with HPV as the primary screening method every 5 years, and also increases the use of self-sampling kits. M Rayner, Healthcare (Basel), 2023.
Colorectal cancer screening in the U.S. typically begins at age 45 and offers multiple options, including annual FIT, colonoscopy every 10 years, and sigmoidoscopy, with screening often done opportunistically during routine care. In Europe, most programs offer screening for people aged from 50 up to 74, with biennial FIT as a primary method, delivered through organized invitations. AI-assisted colonoscopy is also being tested in some countries to enhance detection. M Zhu, Front Oncol, 2025
In the U.S., lung cancer screening is recommended for adults aged 50-80 with a history of at least 20 pack-years of smoking, using low-dose CT as the primary method, but participation remains low despite the guidelines. In Europe, low-dose CT is being piloted in several countries like France, Germany and Italy for high-risk groups, particularly heavy smokers, and is often integrated with smoking cessation programs to improve outcomes. (USPSTF Recommendation, 2021). R Y Kim, JAMA Netw Open, 2025, A Bhamani, Lancet Oncol, 2025.
In the U.S., prostate screening involves shared decision-making, with PSA testing recommended for men aged 55-69. However, the use of PSA is controversial, and recommendations vary based on age and risks. In Europe, screening is more selective and organized, with some countries using PSA followed by MRI and exploring risk-based approaches, including MRI and transperineal biopsy for high-risk individuals. (USPSTF Recommendation, 2018.) S Vacarella, BMJ, 2024. J Hugosson, N Engl J Med, 2022.
Gastric cancer screening is not routinely performed and is limited to individuals identified as high-risk. In the U.S., these screenings typically involve endoscopy and H. pylori testing. In Europe, pilot programs are underway in high-incidence areas like Eastern Europe, using H. pylori testing with endoscopy surveillance to monitor those most vulnerable. National Cancer Institute, PDQ Cancer Information Summaries, 2023. M Leja, Gut, 2024.
Structured Health Systems Facilitate Broad Cancer Screening Access in Europe
Europe’s universal healthcare system removes financial barriers to cancer screening, ensuring broad access across populations. Centralized, organized programs systematically invite all eligible individuals, following standardized protocols that ensure consistency and quality. EU initiatives like the Beating Cancer Plan work to reduce regional disparities, track inequalities, and ensure that at least 90% of eligible individuals are offered screening by 2025, promoting equitable access to early detection, while supporting member states with policy guidance, data sharing, and research to harmonize standards. D Horgan, Healthcare (Basel), 2022.
Screening Access in the USA Influenced by Insurance Coverage and Socioeconomic Status
The U.S. insurance-based healthcare system drives disparities in cancer screening, closely tied to socioeconomic status and coverage. Uninsured, underinsured, and low-income individuals face major barriers — financial, logistical, and informational — that reduce access to screenings. Screening rates for mammography, Pap tests, and colorectal cancer are up to 52% lower in these groups. Additional gaps persist among racial and ethnic minorities and those in socially vulnerable or underserved areas with limited healthcare access. G Zhao, Am J Prev Med, 2024. R A Smith, Cancer Report: Cancer Research for Cancer Prevention, 2020.
Barriers to Screening in the USA and Europe: Cost, Awareness, Access, and Cultural Factors
In the U.S., high out-of-pocket costs, low health literacy, and limited provider access – especially in rural regions – reduce screening rates. Stigma, mistrust, and language issues further discourage participation. In Europe, while costs are lower, funding gaps and low awareness in marginalized and immigrant communities pose challenges. Remote areas face access issues, and social factors such as taboos and limited support also impact screening. AACR Cancer Disparities Progress Report, American Association for Cancer Research, 2024. G Ferraris, Front Psychol, 2024. A Srinath, Health Policy Plan, 2022.
How Do National Policies and Funding Shape Cancer Screening Rates Success in Europe?
National policies and dedicated EU funding are key to Europe’s higher cancer screening rates and quality control – initiatives like Europe’s Beating Cancer Plan and EU4Health support organized, evidence-based screening programs across member states. Centralized national programs follow EU guidelines to ensure consistent quality and equitable access. Data tools like CanScreen-ECIS enable performance monitoring, while the Cancer Inequalities Registry helps address disparities. Strong governance, long-term planning and cross-sector collaboration promote sustainable, innovative, and equitable cancer screening efforts throughout Europe. (CanScreen-ECIS, European Commission, 2023, OECD Health Policy Studies, 2024.)
COVID-19 and Cancer Screening: Disruption and Recovery?
The COVID-19 pandemic significantly disrupted cancer screening in both the U.S. and Europe, with programs paused or slowed and screening rates dropping sharply. In the U.S., screenings dropped by over 80% in April 2020, with uneven recovery and greater impacts on marginalized groups. In Europe, 90% of areas across 34 countries reported interruptions or slowdowns in organized screening programs, delays in diagnosis and reduced lab capacity. Both regions continue to face backlogs, delayed referrals, and resource constraints, with long-term impacts on cancer outcomes still being evaluated. L Allahcoli, Curr Opin Support Palliat Care, 2022
Photo: Depositphotos
Measuring Success: Cancer Screening Outcomes, Effectiveness and Challenges in the U.S. and Europe
Organized cancer screening in Europe significantly reduces mortality and promotes earlier diagnosis. Mortality rates for breast, cervical and colorectal cancers are reduced by 10-91% among those screened. Screening results in a noticeable shift in cancer stages, as seen in breast cancer, where early-stage cases increased by up to 45%, and in cervical cancer, where early microinvasive cancers rose from 33% to 62%. These benefits are driven by structured invitations and quality assurance. However, regional disparities remain, with countries in Eastern Europe continuing to face challenges due to gaps in coverage. S Guthmuller, J Health Icon, 2023. N Zielonke, Eur J Cancer, 2020. J C Teixiera, Sci Rep, 2024. R Cardoso, Lancet Oncol, 2024.
The U.S. initiates cancer screening earlier than many countries, with mammograms starting at age 40 and colorectal screenings at 45. (USPSTF, 2024). USPSTF, 2021. While this helps reduce mortality, it also leads to overdiagnosis (detecting cancers that wouldn’t cause harm if left untreated) – 15-30% of screen-detected cases for breast cancer, rising to over 50% in women older than 85, and 40-60% for prostate cancer through PSA testing, often resulting in unnecessary treatments. I B Richman, Ann Intern Med, 2023. G S Sandhu, J Natl Cancer Inst Monogr, 2012.
Challenges include limited awareness, with only 16% of U.S. women aware of the risks of overdiagnosis, and equity concerns, as earlier screening may benefit some groups while increasing overdiagnosis in others, particularly older adults. R H Nagler, Med Care, 2017.
From False Alarms to Follow-Ups: Managing Screening Risks
Cancer screenings carry risks like false positives, overdiagnosis, and psychological distress. False positives, such as 4.9% for mammograms, and overdiagnosis often lead to unnecessary follow-up tests, anxiety, and invasive procedures like biopsies and surgeries. T White, PLoS One, 2023.
Quality assurance and follow-up protocols differ between the U.S. and Europe. In Europe, centralized, organized screening programs follow strict EU guidelines, ensuring consistent quality and timely follow-up. The U.S. has decentralized, opportunistic screening with variable quality assurance and fragmented systems, leading to inconsistent adherence to guidelines and a lack of coordination. T A Cronan, Praxis Klinische Verhaltensmedizin und Rehabilitation (Practice of Clinical Behavior Medicine and Rehabilitation), 2012. M Arbyn, Ann Oncol, 2010. E J Siegl, Cancer, 2015.
Many cancer screening guidelines lack clear, transparent information about potential harms, making it difficult for patients and clinicians to make fully informed decisions. N Jaber, National Cancer Institute website, 2022. Adressing this gap to support more balanced and personalized decisions requires transparent, quantitative and patient-centred reporting of both benefits and harms in screening guidelines.
In the 1980s, there were doctors, who could make diagnoses by detecting the odor of sweat from the armpits (not a widespread or standard diagnostic method.)
Innovations and Road Ahead for Screening in the USA and Europe
Europe has made significant progress in cervical cancer prevention. The shift to HPV testing has replaced Pap smears, offering higher sensitivity in detecting high-risk HPV strains. Self-sampling kits allow individuals to collect samples at home, increasing participation. Integration of vaccination enhances the overall effectiveness of cervical cancer prevention, moving Europe closer to elimination targets. D Bhatia, Eurohealth, 2025.
Research into liquid biopsies and multi-cancer detection tests is progressing rapidly in the U.S. and Europe. These minimally invasive methods analyze tumor-derived components in body fluids, offering the potential for earlier and more accurate detection. Efforts focus on improving sensitivity, reducing false positives, and improving overall effectiveness. L Ma, Signal Transduct Target Ther, 2024.
Personalized, risk-based cancer screening strategies are becoming more prominent. Europe is using genetic testing to identify people at high risk, and the U.S. is using family history and polygenic risk scores to guide screening decisions. Research focuses on refining screening protocols, incorporating AI, and targeting high-risk individuals to optimize screening efficiency, reduce harms, and enhance early detection. J van der Broek, J Natl Cancer Inst, 2020. L Leitsalu, Eur J Hum Genet, 2021. M Eriksson, Lancet Reg Health Eur, 2023.
Smarter Screening with Telemedicine, Digital Tools and AI
Telemedicine and digital tools are improving screening access and ease. At-home self-sampling devices allow women to comfortably collect samples, increasing participation in cervical cancer screening. Telemedicine systems connect health workers to experts, extending access to rural areas. Digital platforms manage data, send reminders, and ensure timely follow-ups, and AI integration improves diagnostic accuracy and efficiency. These innovations, along with the EU’s screening goals, are helping overcome barriers and improve healthcare for all. B Serrano, Prev Med, 2022. K E Quinley, J Telemed Telecare, 2013. L G Briggs, J Comp Eff Res, 2022. M Eriksson, Lancet Reg Health Eur, 2023.
Is Cross-Continental Learning the Key to Better Cancer Screening?
There is strong political will and growing collaboration between Europe and the U.S. to harmonize cancer screening guidelines and foster cross-continental learning. By combining Europe’s expertise in organized, population-based screening with the U.S.’s strengths in innovation and personalized approaches, both regions can advance evidence-based practices, accelerate the adoption of new technologies, and improve cancer outcomes globally. Office of Cancer Clinical Proteomics Research, National Cancer Institute, 2023.
So, Who Does It Better?
Europe’s model, built around organized, population-based programs, stands out for its focus on equal access and consistent care. With structured outreach and healthcare available to all, it ensures that more people, regardless of income, education, or location, receive timely, routine screenings. This approach promotes fairness and minimizes disparities across different groups. The United States, on the other hand, excels in innovation and individualized care. It tends to adopt new technologies faster and offers more flexibility in when and how people are screened. Its more decentralized system allows care to be tailored to personal preferences and unique risk factors, making it especially responsive to individual needs.
The best way forward is to combine what each does well. Through shared learning, collaboration, and a continued focus on patient needs, we can work toward a more effective, inclusive approach aimed at reducing the burden of cancer and improving outcomes globally.
You Can Also Read How Much Does it Cost to Approve a Drug? EMA vs. FDA Regulations and Timelines by Oncodaily

Written by Seda Adibekyan
FAQ
What cancer screening tests are recommended for my age and gender?
People often want to know which screenings they should get based on their age and sex. For example, breast cancer screening is recommended for women starting at age 45 (with the option to start at 40), cervical cancer screening for people with a cervix, and colorectal cancer screening for adults starting at age 45.
How often should I get cancer screening tests?
The frequency of cancer screenings depends on the type of cancer and individual risk factors. For instance, women aged 45–54 are advised to get mammograms yearly, while those 55 and older can switch to every two years. Colorectal cancer screening can be done every 10 years with a colonoscopy, or more frequently with other tests
What are the benefits and limitations of cancer screening?
Cancer screening helps detect cancer early, before symptoms appear, which can improve treatment outcomes and survival rates. However, there are limitations, such as the risk of false positives, false negatives, discomfort, and anxiety related to the procedure or waiting for results
Where can I get screened and will it be covered by insurance?
Many people are concerned about access and cost. There are resources for those who are uninsured or underinsured to find low-cost or free screenings. It is also important to ask your provider if your insurance will cover the cost of screening tests
What should I expect during a cancer screening test, and how do I prepare?
Patients often want to know what to expect during a screening test and how to prepare. Healthcare providers will explain the procedure, preparation requirements, and what the results can and cannot reveal
Which cancer screenings are recommended in Europe?
Organized screening programmes in Europe are recommended for breast, cervical, and colorectal cancers. These are based on evidence that screening for these cancers can save lives and outweigh potential harms. Other cancer screenings are not currently recommended at the population level.
At what age should I start cancer screening in Europe?
Breast cancer: Women are typically invited starting at age 50 (not before 40), with screenings every 2 years until age 70–75. Cervical cancer: Women start at age 25–30 for cytology (Pap test), or age 35 for HPV testing, with intervals of 3–5 years. Colorectal cancer: Men and women start at age 50–60, with tests every 2 years for faecal tests, or every 10 years for colonoscopy.
How does cancer screening work in Europe?
Screening can be Opportunistic: Performed in clinical settings outside public policy. Programme screening: Publicly funded and part of a public policy. Organised screening: Clearly defined procedures with a dedicated team responsible for implementation. Population-based screening: Eligible individuals are personally invited for each round
Is cancer screening free or covered by health insurance in Europe?
Most EU countries offer organized cancer screening programmes free or at low cost to eligible individuals. The European Health Insurance Card can help cover costs for EU citizens receiving care in another member state.
What are the benefits and risks of cancer screening in Europe?
Screening aims to detect cancer early, improving outcomes and saving lives. However, there are risks such as false positives, false negatives, and potential overdiagnosis. Only cancers with clear evidence of benefit (breast, cervical, colorectal) are included in national programmes to maximize benefit and minimize harm.