Anti-EGFR therapy has become a core component of first-line treatment for RAS/BRAFV600 wild-type metastatic colorectal cancer, particularly in MSS/pMMR disease. However, even within molecularly “eligible” populations, outcomes remain heterogeneous—reflecting additional resistance mechanisms beyond canonical RAS/BRAF alterations.
Among these, HER-2 (ERBB2) gene alterations—including amplification and point mutations—have emerged as biologically plausible drivers of primary resistance to EGFR blockade. While HER-2 amplification is established as a targetable alteration in CRC, its prognostic value and its impact on anti-EGFR efficacy remain debated, and the clinical relevance of HER-2 mutations is even less clearly defined. In this context, results from an exploratory biomarker analysis of the CAPRI-2 GOIM trial were published in ESMO Gastrointestinal Oncology in 2026, evaluating how ERBB2 alterations correlate with response and survival among patients treated with FOLFIRI + cetuximab in the first line.
Title: HER-2 gene alterations as biomarker in patients with metastatic colorectal cancer treated with FOLFIRI + cetuximab: findings from the CAPRI-2 GOIM study
Authors: D. Ciardiello, L. Boscolo Bielo, S. Napolitano, E. Martinelli, T. Troiani, E. Cioli, T.P. Latiano, E. Maiello, P. Parente, A. Avallone, A. De Stefano, R. Bordonaro, A.E. Russo, C. Lotesoriere, S. Vallarelli, S. Pisconti, C. Nisi, E. Tamburini, M.G. Viola, S. Lonardi, C. Cremolini, D. Iacono, P. Tagliaferri, F. Pietrantonio, G. Tortora, G. Rosati, M.G. Zampino, G. Curigliano, A. Febbraro, N. Normanno, F. De Vita, N. Fazio, F. Ciardiello, G. Martini.
Methods
This was an exploratory biomarker analysis within the CAPRI-2 GOIM phase II trial (NCT05312398). Patients with initially RAS/BRAFV600 wild-type, MSS/pMMR mCRC received first-line FOLFIRI plus cetuximab. Before therapy, plasma and tumor tissue were collected for comprehensive genomic profiling using FoundationOne CDx (tissue) and FoundationOne Liquid CDx (plasma).
HER-2 positivity was defined by either ERBB2 amplification (gene copy number ≥4) or ERBB2 mutation detected in tissue and/or liquid biopsy. The analysis examined objective response rate (ORR), progression-free survival (PFS; from treatment start to progression or death), and overall survival (OS), comparing HER-2-positive versus HER-2-negative tumors using Kaplan–Meier methods, log-rank testing, and Cox proportional hazards modeling (exploratory given the small HER-2-positive cohort).
Results
Among 240 screened patients, 192 received first-line therapy and were eligible for evaluation. After exclusions (including tumors with RAS/BRAFV600 pathogenic variants and missing key clinical data), the final cohort included 161 HER-2-negative and 10 HER-2-positive RAS/BRAFV600 WT MSS/pMMR tumors. Overall, ERBB2 alterations were observed in 5.8% of this molecularly selected population and consisted of amplification alone, mutation alone, or combined amplification plus mutation (with reported variants including D769H, R678Q, V842I, and S310F).
Key findings (condensed):
- ORR: 78% (HER-2-negative) vs 60% (HER-2-positive); OR 1.95 (95% CI 0.47–8), P = 0.4.
- PFS: 13.47 vs 7.54 months; HR 2.47 (95% CI 1.27–4.65), P = 0.007.
- OS: 33.4 vs 16.4 months; HR 2.54 (95% CI 1.09–5.93), P = 0.031.
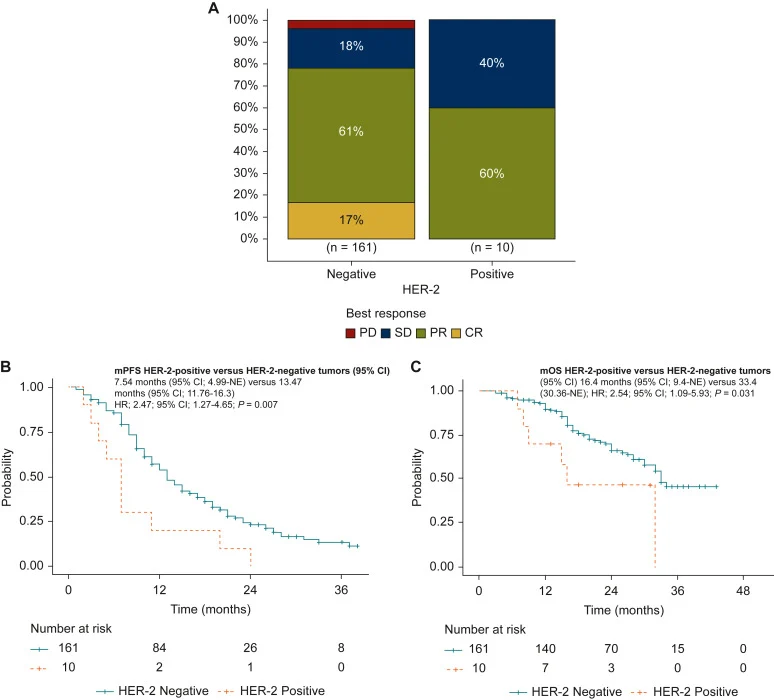
In descriptive subgroup observations, HER-2 mutations were associated with limited durability of benefit, with 6 of 7 cases showing PFS <8 months on FOLFIRI + cetuximab. By contrast, among tumors with ERBB2 amplification without a concurrent mutation, outcomes were more heterogeneous, with 2 of 3 cases exceeding 10 months PFS. The investigators also noted that detection of ERBB2 amplification by liquid biopsy could be limited in samples with low ctDNA tumor fraction, supporting the value of paired tissue assessment when feasible.
Conclusion
In this CAPRI-2 GOIM exploratory analysis, ERBB2 (HER-2) gene alterations were present in 5.8% of RAS/BRAFV600 WT MSS/pMMR mCRC and were associated with significantly worse outcomes, characterized by shorter progression-free and overall survival, on first-line FOLFIRI plus cetuximab. These data support routine baseline assessment of HER-2 genomic alterations—amplifications and mutations—in patients considered for anti-EGFR therapy, both to refine prognostication and to identify individuals who may require alternative strategies, particularly when HER-2 mutations are present.
Full article is available in ESMO Gastrointestinal Oncology.
Read about CAVE-2 GOIM Trial Results: ctDNA-Guided Cetuximab Rechallenge in MSS RAS/BRAF Wild-Type Metastatic Colorectal Cancer on OncoDaily.