Despite major advances in systemic therapy, metastatic colorectal cancer (mCRC) remains challenging to treat once standard options are exhausted. This is particularly true for microsatellite-stable (MSS) tumors, which account for approximately 95% of mCRC cases and show limited sensitivity to immune checkpoint inhibitors. As a result, improving outcomes in refractory MSS mCRC depends largely on optimizing targeted and biomarker-driven strategies.
Rechallenge with anti-EGFR therapy has emerged as a meaningful later-line option in selected patients with RAS/BRAF wild-type mCRC who previously responded to EGFR inhibition and later cleared resistance clones during an anti-EGFR–free interval. The increasing availability of plasma ctDNA comprehensive genomic profiling (CGP) has enabled more refined patient selection beyond RAS and BRAF alone.
The CAVE-2 GOIM randomized phase II trial was conducted to determine whether combining PD-L1 inhibition (avelumab) with cetuximab rechallenge could further improve outcomes in this molecularly selected population. The trial, titled “Cetuximab rechallenge in molecularly selected metastatic colorectal cancer: the randomized CAVE-2 GOIM trial,” was published as an open-access original article in ESMO Annals of Oncology on December 22, 2025.
Authors: D. Ciardiello ∙ G. Martini ∙ F. Pietrantonio ∙ A. Raimondi ∙ P. Manca ∙ S. Pisconti ∙ C. Nisi ∙ G. Tortora ∙ L. Salvatore ∙ A. Sartore-Bianchi ∙ S. Siena ∙ L. Blasi ∙ E. Ongaro ∙ A. Zaniboni ∙ C. Pinto ∙ L. Antonuzzo ∙ A. Avallone ∙ N. Normanno ∙ G. Santabarbara ∙ M.G. Zampino ∙ R. Berardi ∙ A. Cogoni ∙ C. Lotesoriere ∙ T.P. Latiano ∙ E. Maiello ∙ N. Fazio ∙ G. Curigliano ∙ R. Bordonaro ∙ T. Troiani ∙ F. De Vita ∙ E. Martinelli ∙ F. Ciardiello ∙ S. Napolitano
Methods and Endpoints
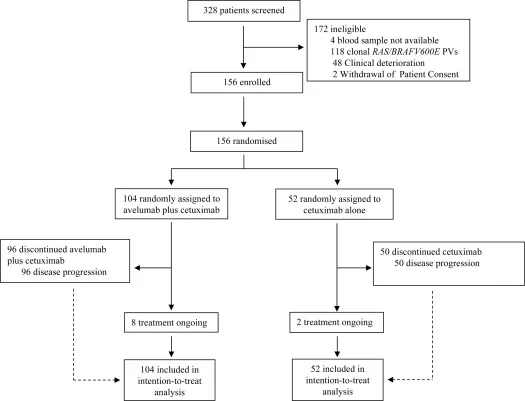
CAVE-2 GOIM (NCT05291156) was an academic, multicenter, open-label, randomized phase II study enrolling patients with refractory MSS mCRC.
Key eligibility criteria included:
- Prior anti-EGFR–based first-line therapy with documented response
- Disease progression followed by at least one anti-EGFR–free line
- Anti-EGFR–free interval of at least 4 months
- Absence of clonal RAS or BRAF V600E mutations on baseline plasma ctDNA (FoundationOne Liquid CDx)
Patients were randomized 2:1 to:
- Cetuximab plus avelumab, or
- Cetuximab monotherapy as anti-EGFR rechallenge
The primary endpoint was overall survival (OS). Secondary endpoints included progression-free survival (PFS), objective response rate (ORR), and safety. Pre-planned exploratory analyses evaluated the impact of extended EGFR-pathway alterations (“molecular hyper-selection”), tumor mutational burden (TMB), and liver metastases.
Results
Between August 2022 and December 2024, 156 of 328 screened patients were randomized (104 to cetuximab plus avelumab and 52 to cetuximab alone). The study population was heavily pretreated, with over one-quarter of patients having received three or more prior lines of therapy.
At a median follow-up of 20.4 months, the addition of avelumab did not translate into a statistically significant survival benefit.
Key efficacy outcomes were as follows:
- Median OS: 14.8 months with cetuximab plus avelumab vs 12.9 months with cetuximab (HR 1.00; P=0.983)
- Median PFS: 5.3 vs 4.3 months, respectively (HR 0.78; P=0.158)
- ORR: 12% vs 8%, respectively
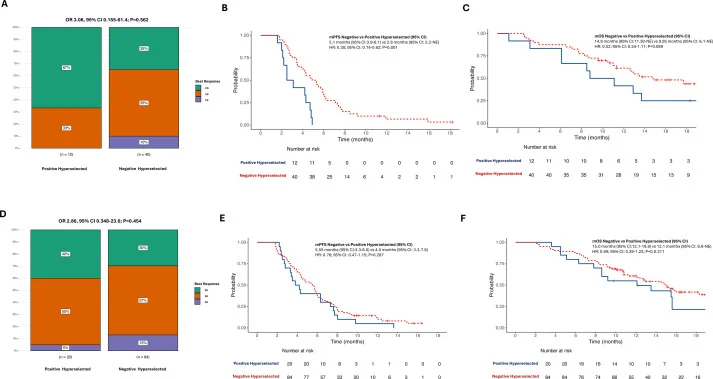
A major strength of the study was the pre-planned extended ctDNA analysis. Patients whose tumors lacked pathogenic alterations in a broad panel of EGFR-pathway resistance genes (“negative hyper-selection”) experienced significantly better outcomes than those with detectable resistance variants:
- Median PFS: 5.35 vs 3.65 months
- Median OS: 15.0 vs 11.1 months
These benefits were observed consistently across both treatment arms, underscoring the prognostic and predictive value of comprehensive ctDNA profiling.
Exploratory subgroup analyses suggested a signal of enhanced activity for cetuximab plus avelumab in patients without liver metastases, including higher response rates and numerically longer PFS and OS, although these findings did not reach statistical significance. In contrast, no benefit from immunotherapy addition was observed in patients with liver involvement.
High tumor mutational burden emerged as a negative prognostic factor in this MSS population, with poor outcomes seen regardless of treatment assignment.
The safety profile was favorable, with no unexpected toxicities or treatment-related discontinuations. Grade ≥3 adverse events were infrequent and primarily related to cetuximab-associated skin toxicity.
Conclusion
The CAVE-2 GOIM trial confirms that adding avelumab to cetuximab rechallenge does not improve survival in molecularly selected MSS RAS/BRAF wild-type metastatic colorectal cancer. However, the study provides compelling prospective evidence that ctDNA-guided patient selection, particularly through extended EGFR-pathway profiling, is a key determinant of benefit from anti-EGFR rechallenge.
These findings reinforce the concept that a biologically defined subset of refractory mCRC remains dependent on EGFR signaling and can achieve clinically meaningful outcomes when treated with appropriately selected targeted therapy.
Read the full article here.