This article offers a comprehensive overview of meningioma, the most common primary brain tumor in adults. It explores key aspects including the tumor’s origin, types, causes, symptoms, diagnostic process, treatment strategies, and the potential for malignancy. Special emphasis is placed on the latest advances as of 2025, including molecular classification, targeted therapies, and immunotherapy research, reflecting the shift toward personalized care in neuro-oncology.
What Is Meningioma?
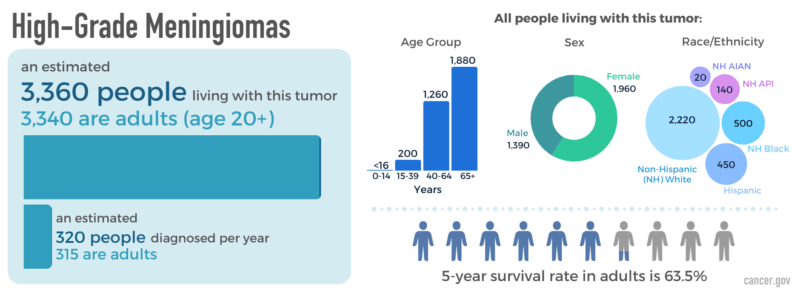
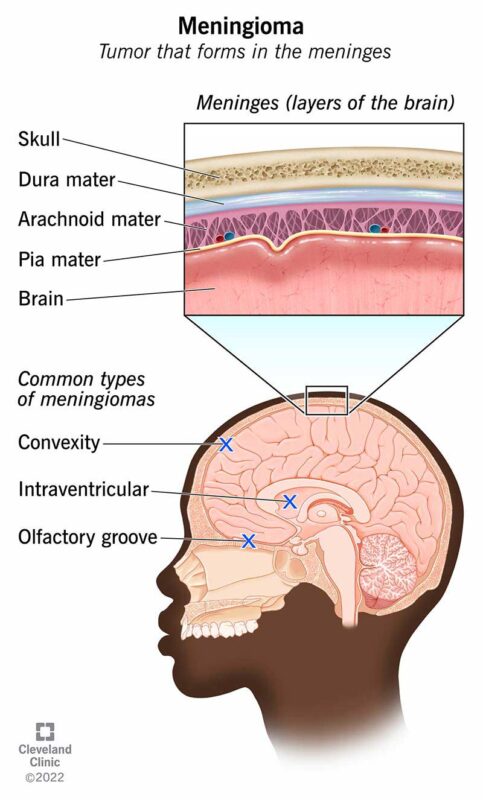
Meningioma is a typically slow-growing, extra-axial tumor that arises from the meninges, the membranous layers surrounding the brain and spinal cord. It is the most common primary intracranial tumor, accounting for approximately 30–40% of such tumors in adults (Louis et al., 2021). Meningiomas originate from arachnoid cap cells and are most often benign (WHO Grade 1), though a subset may be atypical (Grade 2) or anaplastic/malignant (Grade 3), with more aggressive behavior and higher recurrence risk (Weller et al., 2021).
These tumors are more frequently diagnosed in women and typically occur between the ages of 40 and 70. Their pathogenesis is associated with various genetic and environmental factors, including mutations in the NF2 gene, hormone receptor expression, and prior cranial irradiation (Ostrom et al., 2022). While many meningiomas are asymptomatic and discovered incidentally, symptomatic cases may present due to mass effect, depending on tumor size and location.
Meningioma and Malignancy: Understanding the Spectrum
While most meningiomas are benign, a significant subset exhibits malignant behavior. The World Health Organization (WHO) classifies meningiomas into three grades based on histological and molecular criteria: grade 1 (benign), grade 2 (atypical), and grade 3 (anaplastic/malignant). WHO grade 3 meningiomas, though rare (1–3% of cases), are considered malignant due to their high mitotic index, cellular anaplasia, necrosis, and aggressive clinical course (Louis et al., 2021).
Malignant meningiomas tend to recur quickly after resection, often within 1–2 years, and frequently invade brain parenchyma or metastasize extracranially, particularly to the lungs, liver, or bones (Weller et al., 2021). Histologically, they may resemble sarcomas or carcinomas and exhibit rapid proliferation (≥20 mitoses per 10 high-power fields).
At the molecular level, malignant behavior is often associated with alterations such as TERT promoter mutations, CDKN2A/B homozygous deletions, and loss of NF2—all of which are indicators of poor prognosis and resistance to conventional therapies. These molecular markers have been integrated into the 2021 WHO classification to refine diagnosis and risk stratification.
Management of malignant meningioma typically requires gross total resection followed by adjuvant radiotherapy, often with intensity-modulated or proton beam techniques. Given their resistance to standard treatments, these tumors are the focus of ongoing trials investigating targeted agents, immune checkpoint inhibitors, and tumor-treating fields.
In summary, while most meningiomas are benign, a malignant subset exists, defined by WHO grade 3 criteria and characterized by rapid growth, recurrence, and potential metastasis. Early recognition and molecular profiling are essential for optimal management.
Read More About Brain Cancer on Oncodaily
Types and Classification of Meningioma
Meningiomas are categorized by the World Health Organization (WHO) into three grades based on their histopathologic features, recurrence risk, and biological behavior. WHO Grade 1 meningiomas are considered benign and represent approximately 80–90% of all cases. They are slow-growing and have a low likelihood of recurrence after surgical resection. Common histological subtypes within Grade 1 include meningothelial, fibrous (or fibroblastic), transitional (mixed), psammomatous, angiomatous, and microcystic meningiomas (Louis et al., 2021).
WHO Grade 2 meningiomas, known as atypical meningiomas, comprise 5–15% of all meningiomas and are characterized by increased mitotic activity (≥4 mitoses per 10 high-power fields), brain invasion, or particular histological features such as clear cell or chordoid morphology. These tumors have a higher recurrence rate and are more likely to require adjuvant radiation therapy following surgery (Weller et al., 2021).
WHO Grade 3 meningiomas, also called anaplastic or malignant meningiomas, are rare (1–3% of cases) but highly aggressive. They show features such as ≥20 mitoses per 10 high-power fields, necrosis, or poorly differentiated (sarcomatoid, papillary, or rhabdoid) histology. These tumors tend to recur quickly and are associated with poor overall survival, often necessitating combined-modality treatment strategies (Louis et al., 2021).
In addition to histological grading, recent advances have introduced molecular classification frameworks that incorporate genetic alterations such as NF2 loss, TERT promoter mutations, and CDKN2A/B deletions. These molecular features offer additional prognostic value and may influence treatment planning in future personalized approaches (Weller et al., 2021).
Causes of Meningioma
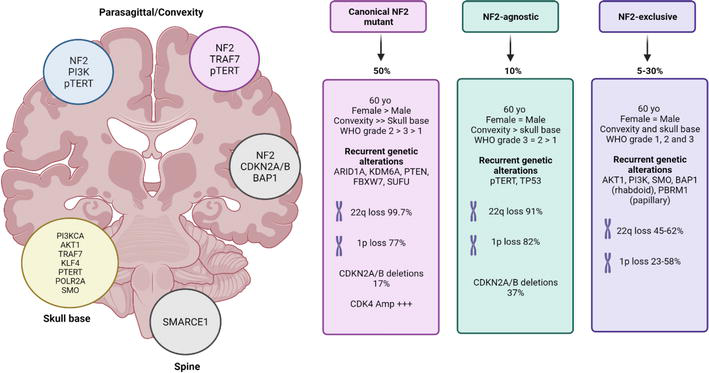
The exact cause of meningioma remains unclear, but both genetic and environmental factors contribute to its development. One of the most well-established genetic contributors is the mutation or loss of the NF2 gene located on chromosome 22. This gene encodes the merlin protein, a tumor suppressor that regulates cell growth. Loss of NF2 function is observed in about 50–60% of sporadic meningiomas, especially in tumors located at the cerebral convexities (Louis et al., 2021).
In addition to NF2 alterations, other somatic mutations have been identified in meningiomas lacking NF2 mutations. These include alterations in TRAF7, KLF4, AKT1, and SMO genes, which tend to be associated with skull base tumors and often correlate with distinct histologic subtypes. These mutations suggest multiple molecular pathways can lead to tumor development (Weller et al., 2021). Environmental risk factors include exposure to ionizing radiation, particularly therapeutic or high-dose diagnostic cranial irradiation, which is a well-documented risk factor for the development of meningioma. Patients exposed to radiation during childhood, such as for tinea capitis or leukemia, have an elevated risk of developing meningiomas later in life (Preston et al., 2007).
Hormonal factors may also play a role. Meningiomas are more common in women, and many tumors express progesterone receptors, suggesting a potential link to hormonal influences. Increased tumor growth during pregnancy and associations with breast cancer have further supported this hypothesis, though causality remains unproven (Ostrom et al., 2022). There is also some evidence linking hereditary cancer syndromes—such as neurofibromatosis type 2 (NF2 syndrome)—to an increased risk of developing multiple or early-onset meningiomas. However, familial cases are rare.
In summary, meningioma development is driven by a combination of genetic mutations, hormonal influences, and environmental exposures, especially ionizing radiation. Ongoing research continues to elucidate the complex molecular landscape of these tumors.
Symptoms of Meningioma
The symptoms of meningioma depend largely on the tumor’s size, growth rate, and location in the brain or spinal cord. Since meningiomas grow slowly and often remain asymptomatic for years, they are sometimes discovered incidentally during imaging for unrelated conditions. However, when symptoms do appear, they result from the tumor compressing adjacent brain tissue, cranial nerves, or vascular structures.
Headaches are among the most common initial complaints, especially with larger tumors or those increasing intracranial pressure. These headaches are often persistent and may worsen in the morning or with changes in body position due to impaired cerebrospinal fluid flow (Weller et al., 2021). Seizures occur in about 20–40% of patients with supratentorial meningiomas, particularly when the tumor is near the cerebral cortex. These may be focal or generalized and are more frequent in tumors located in the frontal or temporal lobes (Louis et al., 2021).
Focal neurological deficits such as limb weakness, sensory changes, or coordination difficulties arise when the tumor exerts pressure on specific brain regions. For example, meningiomas near the motor cortex can lead to hemiparesis, while those near the cerebellum may cause ataxia and balance problems. Visual disturbances are common when the tumor affects the optic nerve or chiasm, often seen in sphenoid wing or tuberculum sellae meningiomas. These may manifest as blurred vision, visual field loss, or even progressive vision loss in one or both eyes.
Cognitive and personality changes, including memory impairment, apathy, or disinhibition, may be seen in tumors affecting the frontal lobes. These changes are often subtle and may be misattributed to psychiatric or degenerative conditions in elderly patients (Ostrom et al., 2022). Cranial nerve dysfunction may occur in skull base meningiomas. Depending on the nerves involved, this can lead to facial numbness, double vision, hearing loss, or difficulty swallowing.
In spinal meningiomas, which are most often found in the thoracic spine, symptoms include back pain, motor weakness, sensory deficits, or bowel/bladder dysfunction due to spinal cord or nerve root compression. Because symptoms can be gradual and non-specific, early recognition is challenging. A high index of suspicion and timely neuroimaging are essential for diagnosis and management.
Diagnosis of Meningioma
The diagnosis of meningioma relies on a combination of clinical evaluation, neuroimaging, and histopathological confirmation. Because these tumors often grow slowly and can remain asymptomatic for years, they are frequently detected incidentally during imaging for unrelated symptoms. When symptomatic, patients usually present with headaches, seizures, visual changes, or focal neurological deficits, prompting further investigation.
Magnetic Resonance Imaging (MRI) is the gold standard for diagnosing meningioma. On MRI, meningiomas typically appear as extra-axial, dural-based masses that are isointense to gray matter on T1-weighted images and hyperintense on T2-weighted images. After contrast administration, they usually demonstrate strong, homogeneous enhancement and may exhibit a characteristic “dural tail” sign, indicating thickening of the dura adjacent to the tumor (Louis et al., 2021). Computed Tomography (CT) is useful in detecting calcifications, which are common in meningiomas, and in evaluating bone involvement, especially for skull base tumors. Hyperostosis of adjacent bone may be seen, suggesting slow, chronic tumor growth.
Histopathologic examination is required for definitive diagnosis and grading. Following surgical resection or biopsy, the tumor is evaluated under the microscope to determine histological subtype and WHO grade (1–3), based on features such as mitotic rate, brain invasion, necrosis, and cellular atypia (Weller et al., 2021). Molecular testing is increasingly used to provide prognostic information and guide future therapeutic strategies. Important molecular alterations include mutations in the NF2 gene (common in non-skull base tumors), TRAF7, AKT1, SMO, KLF4, and TERT promoter mutations. CDKN2A/B homozygous deletions are associated with poor prognosis and are now part of WHO criteria for grade 3 meningiomas (Louis et al., 2021).
Functional imaging, such as positron emission tomography (PET) using tracers like 68Ga-DOTATATE, can help delineate tumor margins or detect recurrence, especially when conventional imaging is inconclusive. This is particularly useful in planning radiosurgery or assessing treatment response. Cerebral angiography may be indicated in select cases where preoperative embolization is considered to reduce intraoperative bleeding, especially in large or highly vascular tumors.
In summary, accurate diagnosis of meningioma depends on high-resolution neuroimaging, histological grading, and, increasingly, molecular characterization to guide personalized treatment and assess prognosis.
Treatment of Meningioma
The treatment of meningioma depends on several factors, including tumor size, location, growth rate, WHO grade, presence of symptoms, and the patient’s overall health. While many meningiomas are benign and asymptomatic, requiring only observation, others necessitate surgical or radiotherapeutic intervention. Observation is appropriate for small, asymptomatic WHO grade 1 meningiomas that are not causing mass effect or edema. Patients are monitored with periodic MRI scans to assess tumor stability. This conservative approach is especially suitable for older individuals or those with comorbidities (Weller et al., 2021).
Surgical resection remains the cornerstone of treatment for symptomatic or growing meningiomas. The extent of resection is a major prognostic factor. Gross total resection (Simpson Grade I–II) significantly reduces recurrence risk and is often curative for WHO grade 1 tumors. However, in tumors located near critical structures such as the cavernous sinus, brainstem, or optic apparatus, subtotal resection may be performed to minimize neurological deficits (Louis et al., 2021).
Radiation therapy is used as an adjunct or alternative to surgery. Stereotactic radiosurgery (SRS) is effective for small to medium-sized tumors, especially in challenging locations. Fractionated radiotherapy is typically employed after subtotal resection or for higher-grade meningiomas (WHO grade 2 or 3) to improve local control and delay recurrence. Adjuvant radiotherapy is strongly recommended for anaplastic meningiomas and often considered for atypical meningiomas depending on surgical margins and mitotic index (Weller et al., 2021).

Read More About Radiotherapy for Brain Tumor on Oncodaily
Medical therapy has a limited role but is under investigation. Hormonal therapies (e.g., anti-progesterone agents) have shown limited benefit. Trials are ongoing for targeted therapies based on molecular alterations, such as AKT1, SMO, or mTOR pathway inhibitors, particularly in recurrent or inoperable tumors. Hydroxyurea and bevacizumab have been explored with modest efficacy in small studies. In recurrent or progressive disease, treatment options include reoperation, re-irradiation, and enrollment in clinical trials evaluating novel agents like somatostatin analogs or immune checkpoint inhibitors. Recurrent WHO grade 3 meningiomas are particularly challenging and often require multimodal approaches.
In summary, meningioma treatment is highly individualized, balancing the goals of maximal tumor control with preservation of neurological function. Advances in molecular profiling may soon lead to more targeted, personalized treatment strategies.
The Role of Immunotherapy in Meningioma
Immunotherapy is an emerging area of research in meningioma treatment, particularly for recurrent or high-grade (WHO grade 2 and 3) tumors, which are associated with higher rates of progression and limited responsiveness to conventional therapies. While no immunotherapy agents are yet approved specifically for meningioma, several studies have identified promising targets and ongoing clinical trials are investigating immune-based strategies.
High-grade meningiomas exhibit greater immunogenic potential than benign subtypes. They tend to show increased expression of immune checkpoint molecules, such as programmed death-ligand 1 (PD-L1), and infiltration of tumor-associated macrophages and T cells, especially in recurrent tumors. These features suggest a potential role for immune checkpoint inhibitors, such as nivolumab (anti–PD-1) and ipilimumab (anti–CTLA-4), in modulating the tumor microenvironment (Weller et al., 2021).
Preclinical studies and small early-phase clinical trials have provided evidence that immune checkpoint blockade may be feasible in meningioma. For example, in a Phase II trial of nivolumab in recurrent grade 2/3 meningioma (NCT02648997), investigators observed some evidence of disease stabilization, though objective responses were limited. Another trial exploring the combination of ipilimumab and nivolumab in high-grade meningioma (NCT03279692) aims to determine whether dual checkpoint inhibition can enhance immune activity and control tumor growth (Magill et al., 2022).
Apart from checkpoint inhibitors, vaccines and adoptive T-cell therapies are under investigation, particularly in tumors expressing somatostatin receptors, TERT promoter mutations, or other neoantigens. Additionally, the use of tumor-infiltrating lymphocyte profiling and molecular sequencing has the potential to identify patients most likely to benefit from immunotherapy. Despite these advances, challenges remain. Many meningiomas, especially WHO grade 1 tumors, are immunologically “cold” with low mutational burden and limited immune cell infiltration, which may hinder the effectiveness of immune-based treatments. Moreover, the lack of standardized immunotherapy biomarkers in meningioma makes patient selection difficult.
In conclusion, while immunotherapy is not yet a standard treatment for meningioma, particularly in the benign setting, it represents a promising approach in recurrent or high-grade disease. Ongoing clinical trials and deeper molecular understanding may soon refine its role within precision oncology strategies.
Read More About Immunotherapy for Brain Cancer on Oncodaily
Meningioma Prognosis: Understanding Factors and Outcomes
The prognosis of meningioma varies significantly based on several key factors, including the tumor’s grade, size, location, molecular characteristics, extent of surgical resection, and patient-specific elements such as age and overall health status.
According to the World Health Organization (WHO) classification, meningiomas are categorized into three grades, each with distinct prognostic implications:
- Grade 1 (Benign): Most common, with generally favorable outcomes. Following complete surgical resection, patients experience excellent long-term survival rates, with over 90% progression-free survival at 5 years and approximately 80–90% survival at 10 years (Louis et al., 2021).
- Grade 2 (Atypical): These tumors exhibit a more aggressive behavior with a higher recurrence risk, estimated around 30–40% within 5 years, even after gross total resection. Adjuvant radiation therapy is frequently recommended to minimize recurrence risk (Weller et al., 2021).
- Grade 3 (Anaplastic/Malignant): Least common but most aggressive, characterized by rapid recurrence and potential metastasis. Median survival ranges between 1.5 and 3 years, with recurrence rates as high as 70–90% despite aggressive multimodal therapy (Louis et al., 2021).
The extent of surgical resection profoundly impacts prognosis. Achieving gross total resection significantly reduces recurrence risk and improves long-term survival, whereas subtotal resection increases recurrence likelihood and can adversely affect outcomes, particularly in atypical or malignant meningiomas. Molecular features also play an important prognostic role. Alterations such as NF2 gene loss, TERT promoter mutations, or CDKN2A/B deletions are associated with more aggressive tumor behavior and poorer prognosis. These biomarkers are increasingly incorporated into routine clinical assessment to help refine prognosis and guide individualized treatment strategies (Magill et al., 2022).
Patient factors including age, functional status, and comorbidities also influence outcomes. Younger patients with good performance status typically achieve better prognoses due to their ability to tolerate aggressive interventions, whereas older patients or those with significant health issues may experience less favorable outcomes. Overall, meningioma prognosis is highly individualized, influenced by a combination of tumor grade, molecular characteristics, extent of resection, and patient-specific factors. Advances in molecular profiling and targeted therapies continue to refine prognostic accuracy and improve long-term management.
Latest 2025 Advances in Meningioma Management
As of 2025, several advancements are shaping the evolving landscape of meningioma diagnosis and treatment, particularly for high-grade and recurrent cases. These innovations span molecular profiling, imaging, radiotherapy, systemic therapies, and immunotherapy—moving the field toward more personalized, risk-adapted care.
Molecular Stratification for Risk-Adapted Therapy
Building on the 2021 WHO CNS classification, new 2025 data further emphasize the prognostic value of specific molecular alterations, such as CDKN2A/B homozygous deletions, TERT promoter mutations, and DNA methylation subtypes, particularly in WHO grade 2 and 3 tumors. These molecular features are now being used in several academic centers to tailor post-operative therapy and surveillance intensity (Louis et al., 2021; Nassiri et al., 2024).
Targeted Therapy Trials
Ongoing clinical trials in 2025 are evaluating targeted therapies based on key mutations in AKT1, SMO, PIK3CA, and NF2. For example, the ACTRUM trial is testing an AKT1 inhibitor in patients with recurrent skull base meningiomas harboring AKT1 E17K mutations, while SMOTRAC is investigating vismodegib for SMO-mutant tumors. These trials are among the first to offer mutation-matched treatment in meningioma (Magill et al., 2022).
Advanced Imaging and Radiomics
2025 studies have validated the use of 68Ga-DOTATATE PET imaging to distinguish aggressive meningiomas from benign subtypes and guide stereotactic radiosurgery planning. Radiomics-based algorithms integrating MRI features and molecular markers are also being used to predict recurrence risk preoperatively, enhancing surgical decision-making and follow-up planning (Weller et al., 2021).
Immunotherapy Updates
While checkpoint inhibitors have shown limited objective responses in past studies, updated 2025 results from the NCT03279692 trial combining nivolumab and ipilimumab in recurrent grade 2/3 meningiomas have demonstrated prolonged disease stabilization in a subset of patients with high PD-L1 expression or immune-active tumor microenvironments. Ongoing studies are exploring combinations of immunotherapy with radiation and oncolytic viruses, seeking to boost T-cell infiltration and immunogenicity.
Liquid Biopsy for Surveillance
Early 2025 results from multi-center cohorts are validating circulating tumor DNA (ctDNA) assays targeting NF2, AKT1, and TERT mutations in plasma and cerebrospinal fluid. These non-invasive tools are being explored for early detection of recurrence, particularly in patients undergoing surveillance after subtotal resection or radiation.
De-escalation in Low-Risk Tumors
New protocols are testing reduced surveillance imaging frequency and delayed radiation strategies for molecularly low-risk WHO grade 1 meningiomas, especially in elderly patients or tumors with indolent radiographic behavior. This approach aims to reduce overtreatment and preserve quality of life.
In summary, the year 2025 marks a shift toward precision medicine in meningioma, with molecularly guided therapies, novel imaging modalities, immunotherapy combinations, and ctDNA-based monitoring beginning to reshape standard of care—especially in patients with atypical or anaplastic tumors.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
FAQ
What is a meningioma?
Meningioma is a type of primary brain tumor that originates from the meninges, the protective membranes surrounding the brain and spinal cord.
Are all meningiomas cancerous?
Most meningiomas (about 80–90%) are benign (non-cancerous). However, atypical (grade 2) and malignant (grade 3) meningiomas exhibit more aggressive behavior.
What causes meningiomas?
The exact cause is unknown, but risk factors include radiation exposure, hormonal influences, genetic mutations (e.g., NF2 gene), and rarely hereditary conditions like Neurofibromatosis type 2.
What symptoms might suggest a meningioma?
Common symptoms include persistent headaches, seizures, visual disturbances, cognitive changes, and focal neurological deficits like weakness or coordination issues, depending on tumor location.
How is a meningioma diagnosed?
Meningiomas are primarily diagnosed through MRI or CT imaging. Definitive diagnosis requires histopathological analysis after surgical biopsy or resection.
What are the treatment options for meningiomas?
Treatment includes observation for small benign tumors, surgery for symptomatic or growing tumors, radiation therapy (e.g., stereotactic radiosurgery), and, rarely, systemic therapies.
Can meningiomas come back after treatment?
Recurrence risk varies by tumor grade and extent of surgical resection. Grade 1 tumors have a low recurrence risk, whereas grade 2 and 3 tumors have significantly higher recurrence rates.
What is the prognosis for patients with meningioma?
Prognosis depends on tumor grade, molecular markers, extent of resection, and patient health. Benign tumors generally have excellent outcomes, while atypical and malignant types carry poorer prognoses.
Is immunotherapy effective for meningioma?
Immunotherapy is currently investigational for meningiomas, particularly high-grade types. Clinical trials evaluating immune checkpoint inhibitors and other immune strategies are ongoing.
What are the latest advances in meningioma treatment in 2025?
Current advances include molecular profiling, targeted therapies matched to genetic mutations, improved imaging with radiomics and PET scans, novel immunotherapy combinations, and liquid biopsy techniques for non-invasive monitoring.