Intrahepatic cholangiocarcinoma (iCCA) remains associated with high postoperative recurrence despite complete (R0) resection. While adjuvant capecitabine has shown benefit in resected biliary tract cancers, outcomes remain suboptimal and optimal postoperative systemic strategies are not well defined. Building on advances in systemic therapy for advanced biliary tract cancer, the ACC trial evaluated whether combining camrelizumab with capecitabine could improve recurrence outcomes after curative surgery. The study was published in The Lancet Gastroenterology & Hepatology (February 2026).
Title: Adjuvant camrelizumab combined with capecitabine in patients with intrahepatic cholangiocarcinoma after surgical resection in China (ACC): a single-arm, single-centre, open-label, phase 2 trial
Authors: Prof. Zheng Wang, Lixing Li, Peiran Huang, Honghu Tu, Xiangyu Zhang, Lei Yu, Fei Liang, Prof. Cheng Huang, Prof. Shuangjian Qiu, Prof. Qinghai Ye, Prof. Zhenbin Ding, Prof. Xiaowu Huang, Prof. Yinghong Shi, Kang Song, Prof. Huichuan Sun, Prof. Xiaoying Wang, Yongfeng Xu, Yao Yu, Prof. Qiang Gao, Prof. Lan Zhang, Prof. Jia Fan, Prof. Jian Zhou.
Study Design and Methods
ACC was a single-arm, single-centre, open-label phase II trial conducted at Zhongshan Hospital, Fudan University (Shanghai, China). Adults aged 18–75 years with pathologically confirmed iCCA who had undergone R0 resection were eligible if they had AJCC 8th edition stage IA (G3) or IB–III disease, ECOG performance status 0–1, no extrahepatic metastases, and adequate organ function.
Treatment started 4–8 weeks after surgery and consisted of eight 21-day cycles of camrelizumab (200 mg intravenously on day 1) plus capecitabine (1250 mg/m² orally twice daily on days 1–14, followed by a 7-day rest). The trial is registered at ClinicalTrials.gov (NCT04295317).
Endpoints
The primary endpoint was recurrence-free survival (RFS) assessed in the full analysis set (patients receiving at least one dose of either drug). Safety was evaluated in the safety set, defined by receipt of treatment and at least one post-baseline safety assessment.
What is Camrelizumab?
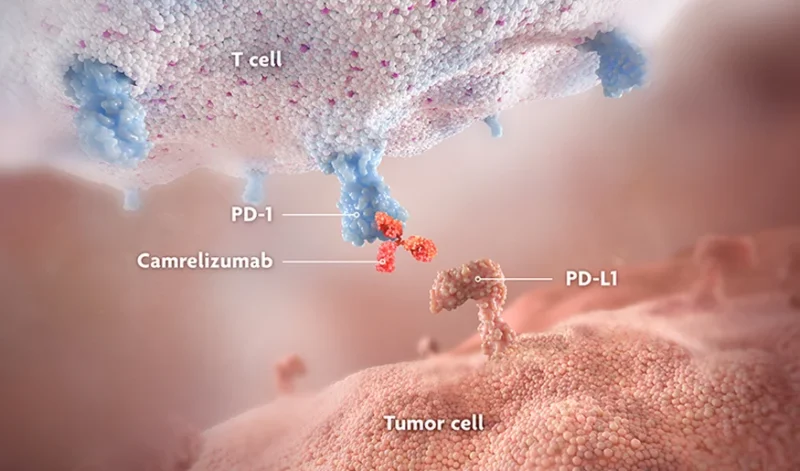
Camrelizumab is a humanized IgG4 monoclonal antibody that targets the programmed cell death-1 (PD-1) receptor, a regulatory protein expressed on activated T lymphocytes. It is approved in China for several malignancies and has been extensively studied in hepatobiliary, gastrointestinal, lung, and nasopharyngeal cancers.
How It Works?
Under normal conditions, the PD-1 pathway functions as a physiological immune checkpoint, limiting excessive immune activation and preventing tissue damage. Many cancers exploit this pathway by engaging PD-1 signaling, thereby suppressing antitumor immune responses.
Camrelizumab works by:
- Binding to the PD-1 receptor on T cells
- Blocking inhibitory signaling mediated through this pathway
- Restoring and sustaining T-cell activity against tumor cells
By releasing this immune “brake,” camrelizumab enhances endogenous antitumor immune surveillance, allowing immune cells to better recognize and eliminate malignant cells.
source: Elervar Therapeutic
Results
Between September 2020 and November 2022, 65 patients were enrolled (median age 64 years; 62% male). At a data cutoff in November 2024, the median follow-up was 33.7 months. Recurrence occurred in 55% of patients, predominantly within the liver.
Median RFS was 24.29 months (95% CI 13.54–not reached). The safety profile was manageable and consistent with known effects of camrelizumab and capecitabine. The most frequent treatment-related adverse events (occurring in ≥10% of patients) were reactive cutaneous capillary endothelial proliferation, nausea, and hand–foot syndrome.
Grade 3 treatment-related adverse events occurred in 23% of patients, most commonly elevated bilirubin, while serious immune-related adverse events were infrequent and included isolated cases of myocarditis, myalgia, type 1 diabetes, and hypothyroidism. Importantly, no grade 4 toxicities or treatment-related deaths were reported.
Conclusion
Adjuvant camrelizumab combined with capecitabine demonstrated encouraging activity and acceptable tolerability in patients with R0-resected intrahepatic cholangiocarcinoma. Although limited by its single-arm design, the ACC trial provides a strong rationale for further evaluation of PD-1–based immunochemotherapy in the adjuvant setting through larger, multicentre randomized studies.
Read the full article here.

Read about Final Results of CARES-310 Study: Camrelizumab + Rivoceranib vs Sorafenib in Unresectable HCC on OncoDaily.