Unresectable hepatocellular carcinoma (HCC) remains a major cause of cancer mortality, particularly in Asia where hepatitis B–related disease is dominant. For years, first-line treatment relied on TKIs such as sorafenib, with limited survival gains. The phase 3 CARES-310 trial tested an immunotherapy–TKI combination, camrelizumab plus rivoceranib (VEGFR2-selective TKI), against sorafenib as first-line therapy for patients with unresectable or metastatic HCC and no prior systemic treatment. Earlier analyses showed significant improvements in progression-free survival (PFS) and overall survival (OS).
Methods and Study Design
CARES-310 was a randomised, open-label, international phase 3 study conducted at 95 sites in 13 countries and regions. Eligible patients were adults with histologically or cytologically confirmed unresectable or metastatic HCC, BCLC B or C not suitable for or progressing after locoregional therapy, Child–Pugh A liver function, ECOG 0–1, and at least one measurable lesion by RECIST v1.1. HBV- and HCV-infected patients received antiviral therapy according to local standards.
Patients were randomised 1:1 to:
- Camrelizumab 200 mg IV every 2 weeks + rivoceranib 250 mg orally once daily
- Sorafenib 400 mg orally twice daily
Randomisation was stratified by macrovascular invasion/extrahepatic metastasis (yes vs no), region (Asia vs non-Asia), and AFP (<400 vs ≥400 ng/mL). Treatment continued until BIRC-confirmed progression, unacceptable toxicity, or withdrawal; treatment beyond progression was allowed if clinically beneficial.
Primary endpoints were OS and PFS (BIRC, RECIST v1.1). Secondary endpoints included ORR, duration of response, disease control, safety, and patient-reported outcomes (EORTC QLQ-C30, QLQ-HCC18, EQ-5D-5L).
You can also read about Sorafenib (Nexavar): Uses in Cancer, Side Effects, Dosage, Expectations, and More on OncoDaily.
What is camrelizumab and how does it work?
Camrelizumab is a cancer immunotherapy known as a PD-1 immune checkpoint inhibitor. It is a humanised monoclonal antibody designed to help the immune system better recognize and attack cancer cells.
Under normal conditions, the PD-1 pathway acts as a “brake” on the immune system, preventing excessive immune activity. Many cancer cells exploit this pathway by expressing PD-L1, which binds to PD-1 on T cells and switches off the immune response. Camrelizumab blocks this interaction, releasing the immune brake and allowing T cells to detect and destroy tumor cells more effectively.
By restoring anti-tumor immune activity, camrelizumab has shown durable clinical benefits across multiple cancers, including hepatocellular carcinoma, especially when combined with anti-angiogenic therapies such as rivoceranib.

Source: Elevar Therapeutics
Results
The results of CARES-310 were published in The Lancet Oncology in December 2025. A total of 543 patients were enrolled: 272 assigned to camrelizumab–rivoceranib and 271 to sorafenib. Most patients were male (84%), Asian (83%), HBV-related (75%), and had macrovascular invasion or extrahepatic metastases (74%). Median follow-up was 22.1 months in the combination arm and 14.9 months with sorafenib.
Overall survival
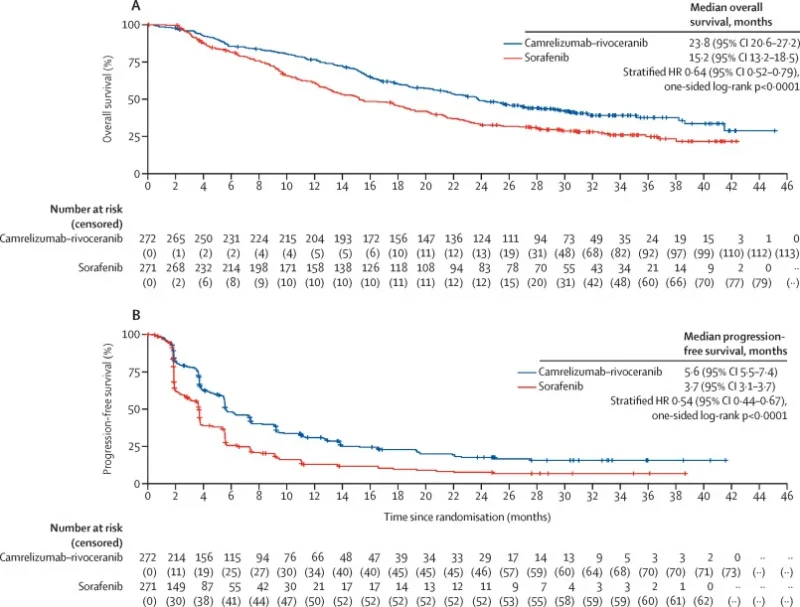
At final analysis (351 deaths, 98% of planned events):
- Median OS: 23.8 months with camrelizumab + rivoceranib vs 15.2 months with sorafenib
- Hazard ratio (HR) for death: 0.64 (95% CI 0.52–0.79; p<0.0001)
Landmark OS rates favoured the combination at 12, 24, and 36 months (for example, 38% vs 25% alive at 36 months). The survival curves separated early and remained apart despite more frequent subsequent systemic therapies, including immunotherapy, in the sorafenib arm.
Progression-free survival and response
By BIRC per RECIST v1.1:
- Median PFS: 5.6 months with camrelizumab–rivoceranib vs 3.7 months with sorafenib
- HR for progression or death: 0.54 (95% CI 0.44–0.67; p<0.0001)
The objective response rate was substantially higher with the combination:
- ORR: 27% vs 6%
- Median duration of response: 17.5 months vs 9.2 months
Responses occurred earlier with camrelizumab–rivoceranib (median time to response 1.9 vs 3.7 months), and deeper tumour shrinkage correlated with longer OS.
Safety and quality of life
Treatment-related adverse events were common in both groups, with more grade 3–4 events in the combination arm, driven largely by VEGFR2-related toxicities:
- Grade ≥3 treatment-related AEs: 81% with camrelizumab–rivoceranib vs 54% with sorafenib
- Most frequent grade 3–4 events with the combination: hypertension, elevated AST and ALT, proteinuria, palmar–plantar erythrodysaesthesia
Treatment-related deaths were rare and similar: one patient in each arm. After adjusting for treatment exposure, the rate of most grade ≥3 events (excluding hypertension) was comparable between groups, suggesting that longer treatment duration and better survival contributed to higher raw event counts.
Immune-related adverse events occurred in 57% of patients on camrelizumab–rivoceranib (17% grade ≥3), mainly laboratory and hepatobiliary abnormalities, and were generally manageable with steroids and treatment modification. Quality-of-life scores were broadly maintained over time, with no major differences between groups despite longer treatment duration in the combination arm.
Conclusion
The final CARES-310 analysis confirms that camrelizumab plus rivoceranib provides a clinically meaningful, durable survival advantage over sorafenib as first-line therapy for unresectable HCC, with:
- Longer overall survival (23.8 vs 15.2 months)
- Improved PFS, response rates, and duration of response
- A manageable, mechanistically expected safety profile, without new late toxicities
- Quality of life broadly maintained during extended treatment
These data consolidate camrelizumab–rivoceranib as a key first-line option for unresectable HCC, particularly in HBV-prevalent populations, and support further evaluation in earlier stages of liver cancer, including in combination with TACE and in the adjuvant setting.
You can read full article here.


