Verzenio® (abemaciclib), in combination with endocrine therapy, has demonstrated significant improvement in overall survival (OS) in patients with hormone receptor-positive (HR+), HER2-negative, node-positive, high-risk early breast cancer (EBC), according to the positive results of the Phase 3 monarchE trial announced by Eli Lilly and Company (NYSE: LLY) on August 27, 2025. This marks a major advancement in the treatment of high-risk EBC, reinforcing the role of Verzenio as the standard of care in this patient population.
Verzenio: A Game-Changer for Node-Positive, High-Risk Early Breast Cancer
MonarchE study’s findings confirm Verzenio’s potential as a standard treatment for HR+, HER2-, node-positive early breast cancer with high recurrence risk. The results also underscore the drug’s differentiated profile in preventing disease relapse and increasing overall survival, even with just two years of therapy.

“Achieving a statistically significant OS benefit with just two years of Verzenio therapy reinforces its differentiated profile in high-risk HR+, HER2- early breast cancer. These data validate Verzenio as the standard-of-care for patients with node-positive, high-risk disease and increase the urgency to ensure all eligible patients are treated.”
Jacob Van Naarden, Executive Vice President and President of Lilly Oncology, said
How Abemaciclib Works: Mechanism of Action
Abemaciclib (brand name Verzenio®) is a CDK4/6 inhibitor that targets cyclin-dependent kinases 4 and 6 (CDK4/6), which are key regulators of the cell cycle. The mechanism of action of abemaciclib involves the inhibition of these kinases, which play a critical role in the transition from the G1 phase to the S phase of the cell cycle. By inhibiting CDK4/6, abemaciclib prevents the phosphorylation of the retinoblastoma protein (Rb), a tumor suppressor protein that controls the progression of the cell cycle.
Key Mechanisms:
- Inhibition of CDK4/6: By blocking CDK4/6, abemaciclib prevents the phosphorylation of retinoblastoma (Rb), which keeps Rb in its active, unphosphorylated state.
- Cell Cycle Arrest: The unphosphorylated Rb binds to and inhibits the E2F transcription factor, which is necessary for the progression of the cell cycle from G1 to S phase. As a result, cell division is halted, leading to cell cycle arrest and inhibition of tumor cell proliferation.
- Selective Targeting of Cancer Cells: While CDK4/6 inhibition affects the cell cycle, its action is particularly relevant in cancer cells that are dependent on these kinases for uncontrolled proliferation, particularly in hormone receptor-positive breast cancer.
- By blocking cell cycle progression, abemaciclib limits the proliferation of cancer cells, especially in cancers such as HR+, HER2- breast cancer, where uncontrolled cell division is a key feature of tumor growth. This mechanism is particularly effective in combination with endocrine therapy, as it enhances the effect of estrogen receptor (ER)-targeted treatments by further suppressing tumor cell proliferation.
About MonarchE Trial
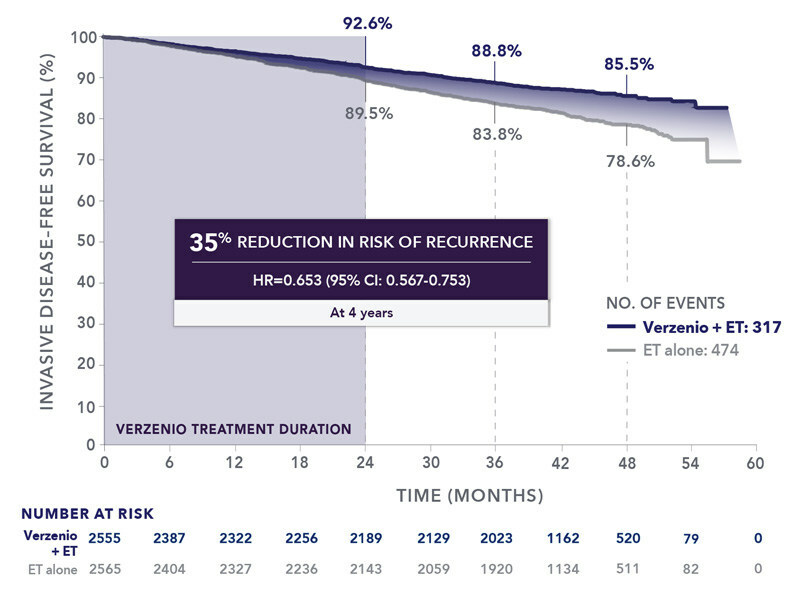
The pivotal monarchE study involved 5,637 adults with HR+, HER2- early breast cancer at high risk of recurrence. These patients were randomized to receive either two years of Verzenio plus endocrine therapy (ET) or ET alone. The trial’s primary endpoint of invasive disease-free survival (IDFS) and overall survival (OS) was evaluated, with Verzenio showing a statistically significant and clinically meaningful improvement in OS compared to ET alone.
In addition to OS, the seven-year landmark analysis revealed sustained benefits in invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS). These results suggest that Verzenio offers durable protection against recurrence and distant metastasis, which is particularly critical in high-risk populations who face an elevated chance of relapse.
Source: investor.lilly.com
Study Design and Methods
monarchE was a global, randomized, open-label, Phase 3 trial, enrolling patients from 600+ sites in 38 countries. The trial was split into two cohorts:
- Cohort 1: 5,120 patients with 4+ positive nodes or 1-3 positive nodes with tumors ≥5 cm or G3.
- Cohort 2: 517 patients with 1-3 positive nodes and a Ki-67 score ≥20%.
Patients in both cohorts were randomized 1:1 to receive either Verzenio 150 mg twice daily plus standard-of-care adjuvant endocrine therapy (Cohort 1: 2,555 patients; Cohort 2: 253 patients) or standard-of-care adjuvant ET alone (Cohort 1: 2,565 patients; Cohort 2: 264 patients) for two years.
This trial is the first adjuvant study designed to investigate a CDK4/6 inhibitor (Verzenio) specifically in a node-positive, high-risk EBC population, a critical area of need for improved treatment outcomes.
Safety Profile of Verzenio in monarchE
The safety profile of Verzenio in the trial remained consistent with previous reports, with no new safety signals emerging during the course of treatment. Diarrhea and neutropenia were the most commonly reported adverse events, consistent with the established safety profile of Verzenio from prior studies.
Notably, Verzenio showed manageable side effects, with most patients experiencing Grade 1 or 2 adverse events. For those with Grade 3 or 4 adverse reactions, dose adjustments were made to minimize side effects, ensuring that treatment could be continued.
The Impact on Early Breast Cancer Treatment
Breast cancer is the second most common cancer worldwide, with HR+, HER2- being the most prevalent subtype of early-stage breast cancer. However, patients with node-positive disease, larger tumors (≥5 cm), and high tumor grades (Grade 3) have a higher risk of recurrence, particularly in the first two years after surgery. The high recurrence rate in these patients underscores the need for more effective treatments, making Verzenio a critical part of adjuvant therapy for this high-risk group.
About Lilly and Oncology Research

For nearly 150 years, Eli Lilly and Company has been at the forefront of pharmaceutical research, with a strong commitment to improving oncology outcomes. The company’s innovations in oncology span biotech and genetic medicine, with a focus on diseases like cancer, diabetes, and Alzheimer’s disease. Through clinical trials like monarchE, Lilly is working to transform cancer care globally by making breakthrough treatments more accessible and effective for patients worldwide.
Conclusion and Next Steps
With Verzenio now established as a standard treatment for high-risk HR+, HER2- early breast cancer, the next steps will include presenting detailed trial results at medical conferences and submitting them for publication in peer-reviewed journals. These findings will also be shared with regulatory bodies to explore further treatment possibilities and approval in additional indications.
You can read the full article here.


