CheckMate 214 Trial Final 9-Year Results at ASCO 2025: Nivolumab Plus Ipilimumab Continues to Show Long-Term Survival Benefit in Advanced Renal Cell Carcinoma
Presented by Dr. Robert J. Motzer at ASCO 2025, the final 9-year follow-up results from the pivotal CheckMate 214 trial confirmed the sustained and durable survival benefit of combining nivolumab and ipilimumab (NIVO+IPI) versus sunitinib (SUN) in patients with advanced renal cell carcinoma (aRCC). These data further reinforce NIVO+IPI as a frontline standard of care, especially for intermediate and poor-risk patients.
CheckMate 214 Trial Design and Patient Enrollment
The phase III CheckMate 214 trial enrolled 1,096 patients with previously untreated, clear-cell advanced RCC. Patients were randomized 1:1 to:
- Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg every 3 weeks for 4 doses, followed by maintenance nivolumab (3 mg/kg or flat doses of 240 mg Q2W or 480 mg Q4W)
- Sunitinib 50 mg orally once daily, 4 weeks on, 2 weeks off
Patients were stratified by International Metastatic RCC Database Consortium (IMDC) risk categories:
- Intermediate/Poor risk (I/P) – Primary analysis population
- Favorable risk (FAV) – Exploratory analysis

Key Results Presented at ASCO 2025
With a median follow-up of 9 years, the CheckMate 214 trial has reinforced the long-term efficacy of the nivolumab plus ipilimumab (NIVO+IPI) combination over sunitinib (SUN) in patients with previously untreated advanced renal cell carcinoma (RCC), across all IMDC risk categories.
Overall Survival
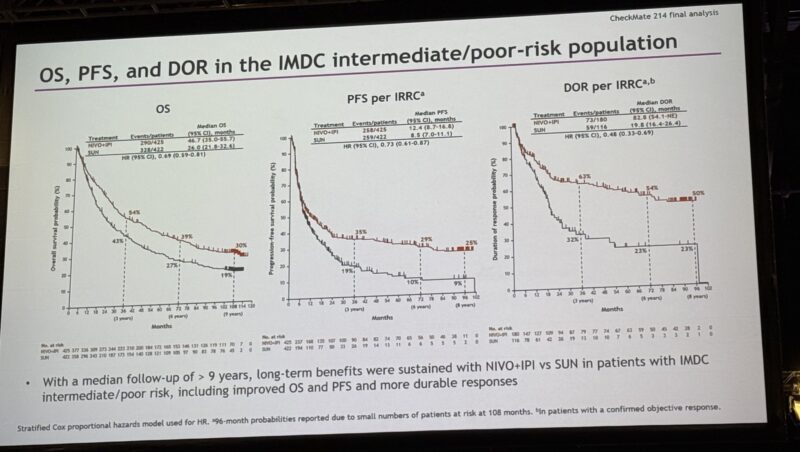
NIVO+IPI continued to demonstrate a sustained survival advantage. In the intent-to-treat (ITT) population, median OS was 53 months compared to 38 months with sunitinib (hazard ratio [HR] 0.71). For intermediate/poor-risk patients, the benefit was even more pronounced—47 vs 26 months (HR 0.69). Favorable-risk patients also saw improvement, with median OS reaching 78 months for NIVO+IPI versus 67 months with SUN (HR 0.80), a notable gain from earlier analyses where the HR was 1.45 in 2018.
Long-term survival rates at 108 months further confirmed these benefits:
- ITT: 31% (NIVO+IPI) vs 20% (SUN)
- Intermediate/Poor-risk: 30% vs 19%
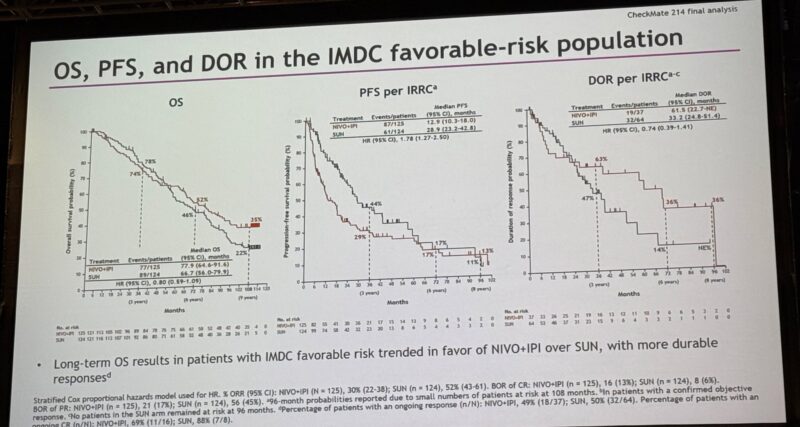
- Favorable-risk: 35% vs 22%
Progression-Free Survival
Median PFS was similar between arms (12 months for both), but long-term disease control significantly favored NIVO+IPI. At 96 months, PFS probabilities were:
- ITT: 23% (NIVO+IPI) vs 9% (SUN)
- Intermediate/Poor-risk: 25% vs 9%
- Favorable-risk: 13% vs 11%
Objective Response Rate
The combination immunotherapy led to higher response rates and more complete responses. In the ITT group, ORR was 39% with NIVO+IPI versus 33% with SUN, including complete response (CR) rates of 12% vs 3%. Intermediate/poor-risk patients saw a 42% ORR and 12% CR with NIVO+IPI, compared to 27% and 3% with SUN. Interestingly, while SUN showed a higher ORR among favorable-risk patients (52% vs 30%), the CR rate still favored NIVO+IPI (13% vs 6%).
Duration of Response
Durability of response was a standout advantage. Among ITT responders, the median DOR with NIVO+IPI was 76 months—triple the 25 months observed with SUN. At 96 months, nearly half of responders remained in remission:
- ITT: 48% (NIVO+IPI) vs 19% (SUN)
- Intermediate/Poor-risk: 50% vs 23%
- Favorable-risk: 36% (NIVO+IPI); not available for SUN
Safety
No new treatment-related deaths were reported in either treatment arm during the extended follow-up. The safety profile remained consistent with earlier reports, with no emerging long-term toxicities.
Read Full Abstract on ASCO Official Website
What People Are Saying About CheckMate 214?
Neeraj Agarwal, MD, FASCO, shared the long-term results of the CheckMate-214 Phase 3 trial on his X page, highlighting the impressive survival rates with immunotherapy in advanced kidney cancer. He wrote:
“In the CheckMate-214 Ph3 trial, it is amazing to see 25% pts (intermediate-poor risk mRCC #kidneycancer) are alive 8 years after starting treatment with ipilimumab+nivolumab”
Key Takeaways from ASCO 2025
Nivolumab plus ipilimumab (NIVO+IPI) continues to stand as a cornerstone first-line therapy for advanced renal cell carcinoma, as reinforced by robust long-term results presented at ASCO 2025. This final 9-year follow-up from the CheckMate 214 trial demonstrated sustained survival benefits across all IMDC risk groups, with particularly striking durability in intermediate and poor-risk patients.
The data represent the longest phase III follow-up of a checkpoint inhibitor combination in RCC to date and emphasize the significant impact of immunotherapy in achieving long-lasting responses. These findings not only support the continued use of NIVO+IPI as a standard of care but also highlight the importance of long-term immuno-oncology data in guiding more personalized treatment strategies for RCC.
More posts featuring ASCO25.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
