The second week of February brings another dynamic wave of advances across GI Oncology, reflecting how rapidly the field continues to evolve at the intersection of regulatory milestones, translational discovery, immunotherapy innovation, and data-driven clinical decision-making.
This week’s highlights span the full spectrum of gastrointestinal malignancies. In pancreatic cancer, we see both regulatory progress with TTFields in locally advanced disease and deep mechanistic insights into KRAS-driven pre-malignant niche programming. In biliary tract cancers, early-phase targeted combinations and awareness initiatives underscore ongoing efforts to expand therapeutic options. Colorectal cancer updates range from peri-operative immunotherapy in MSI-H disease to age-specific analyses of frontline regimens and evolving staging frameworks in hepatocellular carcinoma. Meanwhile, AI-driven pathology tools and immune-modulating strategies in HCC continue to push precision oncology toward more individualized treatment selection.
Together, these expert perspectives illustrate a field that is increasingly biology-informed, technology-enabled, and multidisciplinary in execution—where translational science, clinical trial refinement, and real-world validation are converging to reshape standards of care across GI tumors.
Michael Chuong – Vice Chair, Medical Director, Lead GI Radiation Oncologist – Dept of Radiation Oncology at Miami Cancer Institute
“Exciting news for Novocure and most importantly patients with pancreatic cancer that TTFields received FDA approval for locally advanced pancreatic cancer!!
Our phase 2 trial of TTfields and ablative MR-guided radiation therapy after induction chemotherapy for locally advanced pancreatic cancer is expected to finish accrual later this year!”
Read about PANOVA-3 Leads to FDA Approval of Optune Pax in Locally Advanced Pancreatic Cancer on OncoDaily.
Alexander Kleger – Chairman and Professor of Molecular Oncology, Institute of Molecular Oncology and Stem Cell Biology, University Hospital Ulm
“New paper out in Molecular Cancer (IF 33.9) Springer Nature: oncogenic KRAS doesn’t wait for “cancer” — it starts preparing the niche early.
In this study, we show that KRASG12D in human PSC-derived pancreatic duct-like organoids (PDLOs) triggers an epithelial secretome that
- activates pancreatic stellate cells toward a pro-tumor fibroblast program, and
- drives T-cell exclusion / “shielding” — well before overt PDAC formation.
Importantly, these mechanistic findings are supported by validation in clinical
patient material, strengthening the translational relevance of early niche programming in pancreatic tumorigenesis. Thanks to Stefano Crippa
and colleagues from the Pancreas Center HSRA key mechanistic takeaway: epithelial-derived TNFα emerges as a central ligand in this early niche-preparatory program, linking KRAS signaling to NF-κB/TNF biology and functional immune-access constraints.
Why this matters: understanding pre-malignant niche programming can open doors to earlier interception strategiesand more rational combinations targeting immune exclusion and stromal activation.
And foremost, huge congratulations and thanks to Chantal Allgoewer (first author), who drove this work with exceptional energy and rigor.
Special thanks as well to Markus Breunig (Junior Professor at IMOS and PI in Org-BOOST – Organoid-Based Modelling of Solid Tumors (DFG Research Training Group, Ulm University) for key leadership and continued support.Finally thanks to all our long lasting collaborators and our main funders the Baden-Württemberg Stiftung and Deutsche Forschungsgemeinschaft (DFG) – German Research Foundation”
Jenny Seligmann – Professor of Gastrointestinal Oncology at University of Leeds
“FOxTROT 5 testing Péri-operative dostarlimab in MSI-H locally advanced colon cancer in older or frail patients.
Also thanks to our fantastic investigators.
FOxTROT 6 to follow soon….”

Luis Felipe Leite da Silva – Medical Student | Brazil
“Available on ESMO Open, you will find a synthesis of the current evidence and gaps regarding neoadjuvant immune checkpoint inhibitors in localized dMMR/MSI-H gastric and GEJ cancers”
Anna Berkenblit – Chief Scientific & Medical Officer PanCAN | Biotech Executive | Oncologist | Drug Developer
“Vitara Pancreas ChemoPredict, a new AI diagnostic developed by Valar Labs, was validated using data from patients with advanced pancreaticcancer from two prospective cohorts — PanCAN’s Know Your Tumor® initiative (accessed via the SPARK health data platform) and the COMPASS clinical trial — and analyzes standard H&E-stained pathology slides to identify histological signatures associated with treatment response. This tool will be made available to oncologists through early access to help guide optimal first-line treatment selection for patients with advanced pancreatic cancer.
This work reflects how AI has the potential to move the field forward by helping guide treatment decisions. Read more from our partners at Valar Labs, a leader in computational histology and precision oncology, about the publication in the Journal of Clinical Oncology (JCO).
Special shout outs to PanCAN Scientific and Medical Advisory Board member Andrew Hendifar and PanCAN colleagues Kawther Abdilleh, PhD and Sudheer Doss Ph.D. for their work on this fruitful collaboration!”
Erman Akkus – MD, Medical Oncology, Internal Medicine, Ankara University, Sharing & discussing on cancer, GI oncology
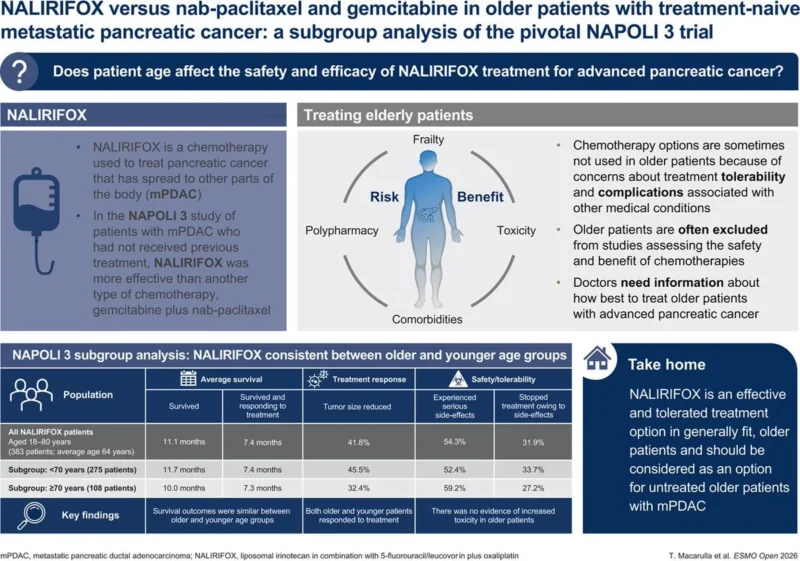
“NALIRIFOX vs Gem-nabpaclitaxel in >70 y patients
subgroup analysis of NAPOLI 3Benefit sustained
No signal for increased toxicity”
Patricia Rider – PhD student at Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) – Hepatocellular Signaling and Cancer group
“I’m excited to share our latest work, now available in Journal of Experimental & Clinical Cancer Research with Springer Nature!
In this study, we investigated the immunomodulatory effects of cabozantinib, a tyrosine kinase inhibitor used in advanced hepatocellular carcinoma (HCC).
At the mechanistic level, cabozantinib induces mitochondrial depolarization and oxidative stress, leading to the release of mitochondrial DNA and subsequent activation of the innate immune pathway cGAS/STING. This leads to the upregulation of type I interferon–stimulated genes, boosting immune activation.
In vivo, these effects translated into reduced tumor growth and enhanced immune responses, especially when cabozantinib was combined with the STING agonist DMXAA, suggesting a promising combinatorial strategy.
Importantly, patient serum proteomics revealed shifts in immune- and stress-related proteins that correlated with clinical outcomes, reinforcing the translational and clinical relevance of our findings.
Overall, this work highlights how targeted therapies can modulate the tumor–immune dialogue and may help guide future therapeutic strategies in HCC.
This study represents a major milestone in my PhD journey. Grateful to all collaborators and mentors who made this work possible!”
Nancy Ghattas – Vice President, US Oncology Commercial Franchise Head – Immuno Oncology
“February is Gallbladder and Bile Duct Cancer Awareness Month and people facing these rare diseases, like cholangiocarcinoma (CCA), need our support more than ever. This disease is challenging to diagnose and difficult to treat, with limited treatment options for patients. But our AstraZeneca team is standing alongside the CCA community, pushing the boundaries of science to offer new therapies and improving patient outcomes.
That commitment has led to more discovery and more potential treatment options in our portfolio and pipeline than ever before. Raising awareness, delivering effective medicines, and supporting patients – we won’t stop until we’ve achieved our ambition to eliminate CCA as a cause of death.”

Mark Yarchoan – Physician scientist, Johns Hopkins, focused on translational immunotherapy research for HCC and cholangiocarcinoma
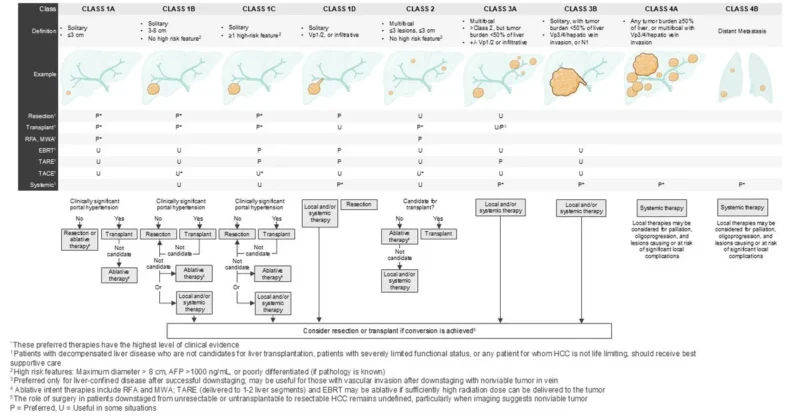
“Barcelona HCC classification may not reflect current treatment practices in US
The new BEACON-HCC Classification was introduced for comment today
HCC LIVE Conference: please weigh in!
Pilot real-world cases: Expert concordance 100% with BEACON-HCC vs 58.6% with 2022 BCLC”
Fen Saj – Research Fellow, Medical Oncologist
“Glad to share our paper now published in Investigational New Drugs, reporting findings from a Phase 1 trial led by Shubham Pant evaluating first-line ivosidenib/pemigatinib combined with gem/cis for advanced cholangiocarcinoma.
The study identified a challenging safety profile, warranting further investigation with dose optimization and integration into current chemoimmunotherapy regimens.
Sincere thanks to co-investigators Tanios Bekaii-Saab, Milind Javle, Joseph Larson, and Mitesh Borad, M.D. for their valuable contributions.”

Find out 10 Must-Read Posts in GI Oncology from the first week of February on OncoDaily.



