On February 11, 2026, Novocure (NASDAQ: NVCR) announced that the U.S. Food and Drug Administration (FDA) approved Optune Pax® in combination with gemcitabine and nab-paclitaxel for the treatment of adult patients with locally advanced pancreatic cancer.
Optune Pax concomitant with gemcitabine and nab-paclitaxel is the first treatment to receive FDA approval in nearly 30 years for locally advanced pancreatic cancer.
Frank Leonard, CEO of Novocure, said:
“The FDA approval of Optune Pax marks the first new treatment in decades for people living with locally advanced pancreatic cancer. Systemic therapies have shown poor bioavailability in pancreatic tumors, limiting their effectiveness. Optune Pax is a fundamentally different treatment, utilizing a biophysical approach that targets the unique electrical properties of cancer cells. This is a proud moment for Novocure and we look forward to bringing Optune Pax to patients and the healthcare providers who care for them.”

Frank Leonard, CEO of Novocure
What Makes Optune Pax Different?
Optune Pax is not a drug. It is a wearable, portable medical device that delivers Tumor Treating Fields (TTFields) non-invasively through wearable arrays. TTFields are alternating electric fields that target the electrical properties of cancer cells to disrupt processes critical for cancer cell division and survival, resulting in cell death without significantly affecting healthy cells.
In preclinical models, TTFields have been shown to disrupt mitosis in cancer cells by exerting electric forces on their polar components (e.g., microtubule spindle formation during mitosis), ultimately leading to cell death. During metaphase, TTFields impair microtubule assembly, leading to aberrant mitotic spindle formation. During anaphase, TTFields disrupt the arrangement of septin at the anaphase cleavage furrow, inducing cytoplasmic membrane blebbing, mitotic failure, and asymmetric chromosome segregation. During telophase, TTFields push polar organelles and macromolecules (a process called dielectrophoresis) to the bridge between the forming daughter cells.
In preclinical models, TTFields have been shown to alter the organization and dynamics of the cytoskeleton, disrupting cancer cell motility and migration. In preclinical models, TTFields-mediated cell disruption has been shown to activate the immune system and induce a downstream immunogenic anti-tumor response, and TTFields have been shown to downregulate DNA damage response pathways.
Due to its multi-mechanistic actions, TTFields therapy can be added to cancer treatment modalities in approved indications and demonstrates enhanced effects across solid tumor types when used with chemotherapy, radiotherapy, immune checkpoint inhibition, or targeted therapies in preclinical models.
Vincent Picozzi, M.D., MMM, medical oncologist and investigator in the PANOVA-3 trial, said:
“In the Phase 3 PANOVA-3 trial, treatment with Optune Pax resulted in a statistically significant improvement in overall survival without adding to the systemic side effects commonly associated with existing therapies. It also significantly extended time to pain progression, helping to preserve overall quality of life, which is a priority when I am treating patients living with pancreatic cancer. With FDA approval, Optune Pax has the potential to be practice changing for the treatment of patients with locally advanced pancreatic cancer.”
What Is Behind the FDA Approval?
The approval is based on the international, prospective, randomized, open-label, controlled Phase 3 PANOVA-3 trial, which results were published in the Journal of Clinical Oncology in 2025.
Anna Berkenblit, MD, MMSc, PanCAN’s Chief Scientific and Medical Officer, said:
“The approval of Optune Pax is an important milestone for the pancreatic cancer community. Survival rates for pancreatic cancer have seen only modest improvements over time and treatment advances have remained limited, underscoring how challenging this disease is to treat. This approval for locally advanced disease highlights the importance of continued innovation and investment in new approaches for difficult-to-treat cancers and represents meaningful progress for patients who urgently need more options.”
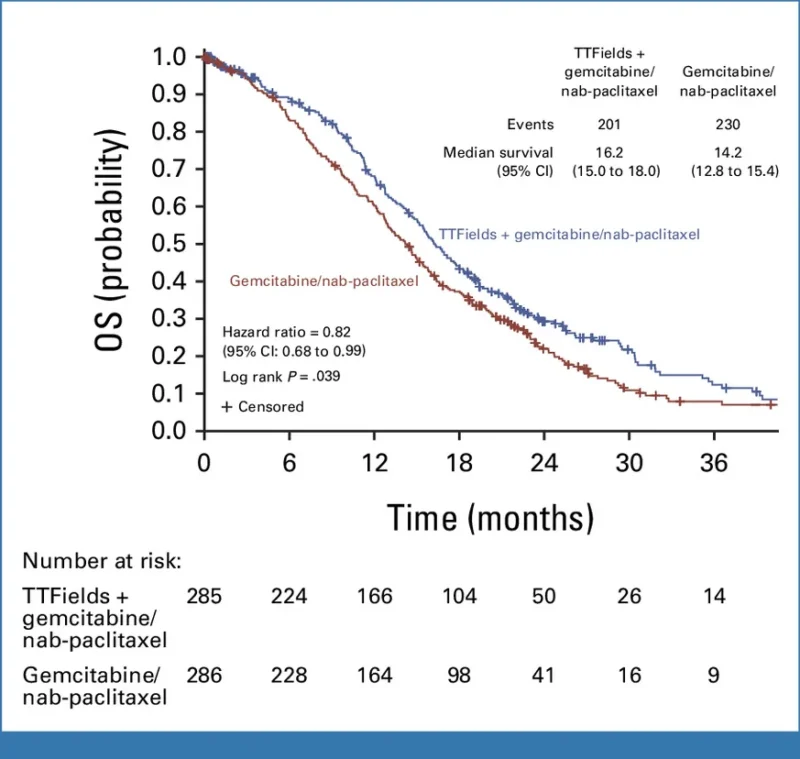
The study evaluated Optune Pax in combination with gemcitabine and nab-paclitaxel (gem/nab-pac) as first-line therapy for locally advanced pancreatic cancer compared with gem/nab-pac alone. It enrolled 571 patients randomized 1:1 and followed them for a minimum of 18 months. It met its primary endpoint, demonstrating a statistically significant improvement in median overall survival.
Overall Survival
In the intent-to-treat population:
- Optune Pax + gem/nab-pac (n=285): 16.2 months (95% CI 15.0–18.0)
- Gem/nab-pac alone (n=286): 14.2 months (95% CI 12.8–15.4)
- Hazard ratio: 0.82 (95% CI 0.68–0.99), p=0.039
This represents a 2.0-month improvement in median overall survival.
In the modified per protocol population:
- Optune Pax + gem/nab-pac (n=198): 18.3 months (95% CI 16.1–20.0)
- Gem/nab-pac alone (n=207): 15.1 months (95% CI 13.4–17.0)
- Hazard ratio: 0.77 (95% CI 0.62–0.97), p=0.023
Here, the improvement reached 3.2 months.
One-Year Survival
One-year survival rates showed a significant improvement in the Optune Pax concomitant with gem/nab-pac group:
- ITT population: 68.1% (95% CI 62.0–73.5) vs 60.2% (95% CI 54.2–65.7)
- Modified per protocol population: 75.2% (95% CI 68.5–80.7) vs 65.9% (95% CI 59.0–72.0)
Read about ESMO GI 2025 Highlights: PANOVA-3 Trial on TTFields + GnP in Unresectable LAPC on OncoDaily.
Pain Control and Quality of Life
Pain progression is a major clinical burden in pancreatic cancer. In PANOVA-3, time to pain progression was defined as the time from baseline until an increase of 20 or more points on a visual pain scale or death.
Median time to pain progression:
- Optune Pax + gem/nab-pac: 15.2 months (95% CI 10.3–22.8)
- Gem/nab-pac alone: 9.1 months (95% CI 7.4–12.7)
This is a 6.1-month extension.
Quality of life was assessed using the EORTC QLQ-C30 and the pancreatic cancer-specific PAN26 addendum. Treatment with Optune Pax resulted in longer deterioration-free survival in global health status, pain, pancreatic pain and most digestive problems. Similar trends were observed for emotional function and fatigue.
No significant differences were observed in progression-free survival, local progression-free survival, objective response rate, puncture-free survival or tumor resectability rate.
Safety Profile
In the Phase 3 PANOVA-3 trial, Optune Pax was generally well tolerated and did not worsen the systemic side effects associated with gemcitabine and nab-paclitaxel. No new safety signals were identified, and serious adverse events were comparable between the treatment groups.
Skin reactions beneath the transducer arrays were common, occurring in 76.3% of patients treated with Optune Pax. Most of these events were mild to moderate (Grade 1–2), while 7.7% of patients experienced Grade 3 or higher skin events. The most frequently reported non-skin device-related side effect was fatigue, affecting 5.1% of participants.
There was one Grade 4 adverse event—non-serious neutrophil count decrease—that was considered by the investigator to be related to the device. Importantly, no device-related adverse events resulted in death, and no unexpected safety concerns emerged during the course of the study.
Who should avoid Optune Pax?
Optune Pax should not be used by patients who are pregnant or planning to become pregnant. If you are a woman who is able to become pregnant, you must use birth control while using the device, as it is not known whether Optune Pax is safe or effective during pregnancy.
Patients with an electrical implant should talk to their doctor before using Optune Pax, because use together with electrical implants has not been tested and may cause the implanted device not to work properly. Patients with a known sensitivity to gels such as those used on ECG stickers or TENS electrodes should also talk to their doctor, as skin contact with the gel used with Optune Pax may commonly cause increased redness and itching and, in rare cases, may lead to severe allergic reactions.
What are the common side effects?
When Optune Pax is used together with chemotherapy, the most common side effects reported include low neutrophils, low red blood cell count, low platelet count, low white blood cell count, diarrhea, nausea, vomiting, abdominal pain, constipation, fatigue, swelling, fever, pain, COVID-19, infection, respiratory tract infection, urinary tract infection, pneumonia, increased liver enzymes, weight loss, low potassium level, low albumin level, high blood sugar, muscle pain, peripheral neuropathy, taste disorder, dizziness, difficulty sleeping, shortness of breath, hair loss, skin-related disorders, and low blood pressure.
Device-related skin side effects can include skin inflammation, rash, itching, skin redness, skin irritation, skin infection, heavy sweating, and open sores. Other device-related side effects may include overheating of the array leading to pain and/or local skin burns, allergic reaction to the adhesive or gel from the transducer arrays, and local warmth and tingling sensation beneath the arrays.
The full FDA approval announcement is available on Novocure’s official website.
Conclusion
Pancreatic cancer remains one of the most lethal malignancies in the United States, with approximately 67,000 new cases diagnosed annually and a five-year relative survival rate of 13%. Incidence and mortality rates continue to rise.
The FDA approval of Optune Pax introduces a new, device-based treatment approach for locally advanced pancreatic cancer, supported by a statistically significant improvement in overall survival and a significant extension in time to pain progression in the Phase 3 PANOVA-3 trial.
Read more about Top 10 Pancreatic Cancer Updates – January 2026 on OncoDaily.