The PANOVA-3 trial (NCT03377491), presented by Dr. Teresa Macarulla Mercade from Barcelona, Spain, at ESMO GI 2025, evaluated the impact of tumor treating fields (TTFields) therapy combined with gemcitabine/nab-paclitaxel (GnP) in patients with unresectable locally advanced pancreatic cancer (LAPC). This phase 3 trial not only demonstrated significant improvements in overall survival (OS) and pain-free survival (PFS) but also assessed the effect of TTFields on patient-reported quality of life (QoL), symptom control, and the use of pain medications. The following results highlight how TTFields therapy, when added to GnP, contributed to better pain management, symptom control, and QoL for patients with this challenging cancer.

Background
In patients with unresectable locally advanced pancreatic cancer (LAPC), treatment options are limited, and the prognosis is generally poor. The PANOVA-3 Phase 3 trial (NCT03377491) evaluated the combination of tumor treating fields (TTFields) therapy with gemcitabine/nab-paclitaxel (GnP) compared to GnP alone. TTFields are low-intensity electric fields delivered through a portable device worn by the patient, which disrupts cancer cell division.
The PANOVA-3 trial demonstrated a significant improvement in overall survival (OS) and pain-free survival (PFS) with the addition of TTFields to GnP. Specifically, the median OS was 16.2 months for the TTFields + GnP arm versus 14.2 months for GnP alone (hazard ratio [HR] 0.82, p=0.039), and the median PFS was 15.2 months versus 9.1 months (HR 0.74, p=0.027). These results suggested that TTFields could improve survival outcomes in patients with LAPC.
This study also sought to evaluate the impact of TTFields on patient-reported quality of life (QoL), pancreatic cancer symptoms, and the use of pain medications, which are critical aspects of managing the disease. Managing symptoms and maintaining QoL are crucial for patients with advanced cancer, and improvements in these areas could have a meaningful effect on patient care and overall well-being. The current analysis thus focuses on the effect of TTFields therapy on symptom management, pain control, and the use of pain medications, alongside the established survival benefits.
Methods
The PANOVA-3 trial randomized patients with unresectable LAPC to receive either the combination of TTFields therapy with GnP or GnP alone in a 1:1 ratio. The QoL was assessed using the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire and the PAN26 module, both of which evaluate various dimensions of physical and functional health as well as disease-related symptoms. Additionally, health-related QoL deterioration-free survival (DFS) was calculated, with a focus on the time until significant deterioration in global health status and key symptoms such as pain.

A post-hoc analysis also evaluated the time to the use of pain medication, specifically assessing the duration until patients began using meaningful pain medications and opioids. The Kaplan-Meier methodology was used for time-to-event analyses, and the log-rank test was employed to compare differences between the treatment arms.
Results
The PANOVA-3 trial evaluated the impact of tumor treating fields (TTFields) therapy when combined with gemcitabine/nab-paclitaxel (GnP) in patients with unresectable locally advanced pancreatic cancer (LAPC). In addition to the significant improvements in overall survival (OS) and pain-free survival (PFS) observed in the trial, this analysis also assessed the effect of TTFields on patient-reported quality of life (QoL), symptom control, and the need for pain medications.
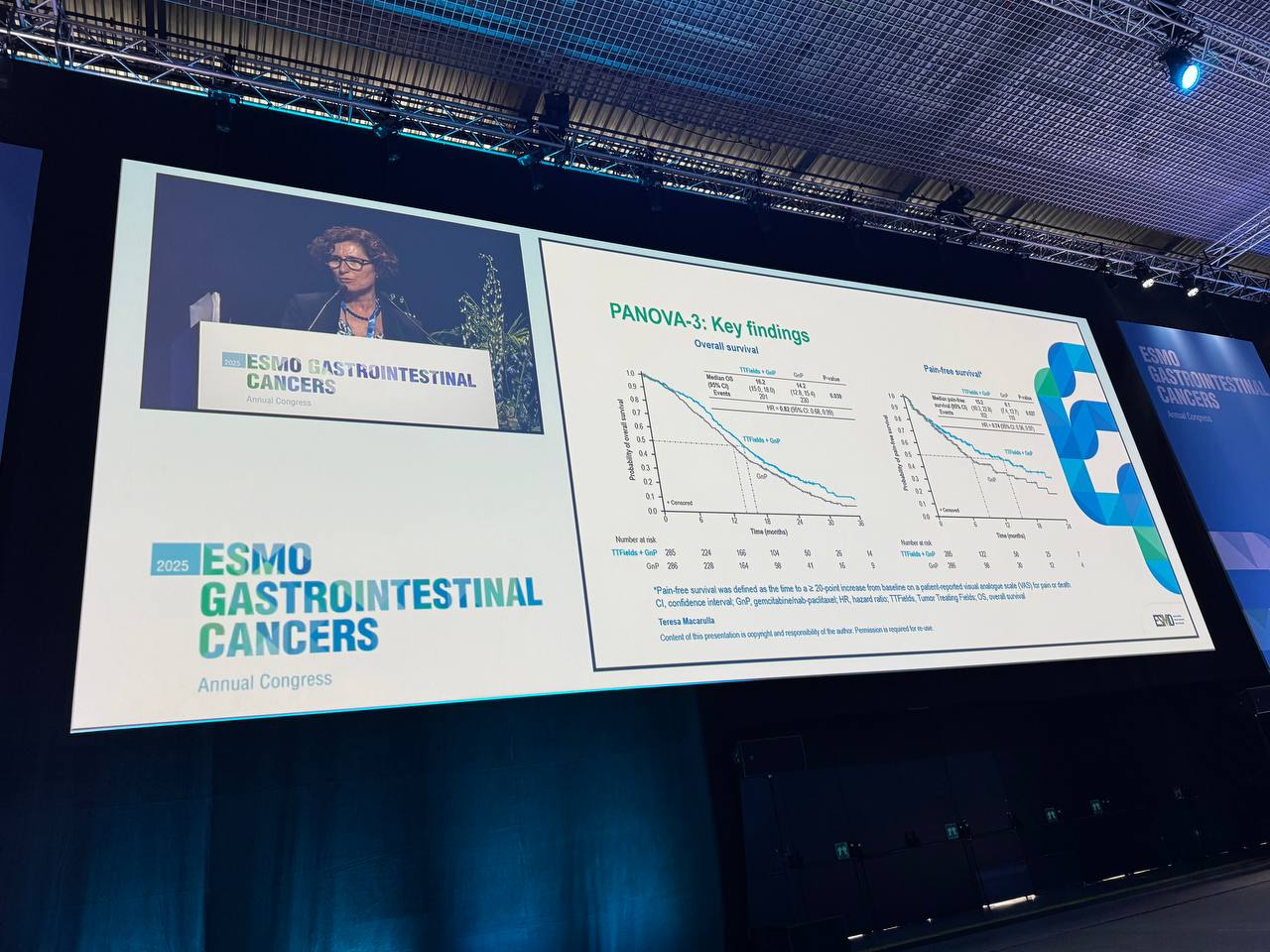
Below are the key findings from the trial regarding these secondary endpoints:
Median time to deterioration of pain:
- TTFields + GnP: 10.1 months
- GnP alone: 7.4 months
- p=0.003
Median time to deterioration of pancreatic pain:
- TTFields + GnP: 14.7 months
- GnP alone: 10.2 months
- p=0.006
Insomnia and Fatigue:
- No significant difference between TTFields + GnP and GnP alone.
Median time to meaningful pain medication use:
- TTFields + GnP: 6.9 months
- GnP alone: 4.9 months
- p=0.049
Median time to opioid use:
- TTFields + GnP: 7.1 months
- GnP alone: 5.4 months
- p=0.046
Digestive Problems:
- All digestive symptom scales, except for indigestion, significantly favored the TTFields + GnP arm.
Median time to deterioration in global health status:
- TTFields + GnP: Significantly longer (p=0.023) than GnP alone.
Functional Scales:
- Trends favored TTFields + GnP, particularly in physical and role functioning, although role functioning favored GnP alone.

Conclusions
The PANOVA-3 trial results demonstrate that the addition of TTFields therapy to GnP significantly improves pain control and quality of life in patients with unresectable LAPC. The combination treatment significantly extended the time to deterioration in both pain and pancreatic pain symptoms, and it delayed the need for pain medications, including opioids. Furthermore, the addition of TTFields therapy to GnP improved global health status, offering patients a better quality of life without compromising other aspects of symptom control.
These findings complement the previously reported overall survival and pain-free survival benefits of TTFields therapy in this patient population, supporting the use of TTFields in combination with GnP as a promising new therapeutic option for patients with LAPC.
You can read the full abstract here


