The ESMO 2025 Congress is a major global oncology event organized by the European Society for Medical Oncology (ESMO).
It is taking place at Messe Berlin in Berlin, Germany, from October 17 to 21, 2025. The congress features a comprehensive scientific and educational program designed to foster exchange and debate in translational cancer science, showcasing potentially practice-changing data, and stimulating multidisciplinary discussions to improve cancer treatment options.
“Many practice-changing studies were presented at this year’s ESMO. Alongside them, several ‘negative’ trials too. To all patients who took part in these studies, a heartfelt thank you. Better cancer care is still ahead, and your courage lights the way.”
Proceed to the video attached to the post.
“Very proud and happy to share that our work on neoadjuvant IO in pMMR colon cancers has been published online in Nature
after presentation at ESMO25. Read on how genomic instability, P53mt, and proliferation may aid in predicting responses.
What changes do we see in the TME post-IO? Does it differ between responders and nonresponders? Tumor reactive phenotypes, and not quantity, of T cells enriched in responders as observed in bulk RNAseq, imaging mass cytometry, and single cell RNAseq.
Stay tuned for a detailed tweet. First, rounding up the last day of ESMO.”
Title: Neoadjuvant immunotherapy in mismatch-repair-proficient colon cancers
Authors: Pedro B. Tan, Yara L. Verschoor, José G. van den Berg, Sara Balduzzi, Niels F. M. Kok, Marieke E. Ijsselsteijn, Kat Moore, Adham Jurdi, Antony Tin, Paulien Kaptein, Monique E. van Leerdam, John B. A. G. Haanen, Emile E. Voest, Noel F. C. C. de Miranda, Ton N. Schumacher, Lodewyk F. A. Wessels, Myriam Chalabi
Read more in Nature.

“ESMO25 had 4 GU trials in the Presidential plenary sessions!
On behalf of our GU team, we were proud to represent the Cleveland Clinic MD for three-quarters of the year!
IMvigor011, KN-905, and PSMAddition! Truly grateful to patients and families, my co-investigators, and clinical research team for helping us make meaningful contributions to these practice-changing trials!”

“Just back from ESMO25!
Latest in glioma Rx:
- SCOUT trial’s ‘watch-and-see’ NTRK inhibitor approach (challenge: when to pause?)
- IL13Ra2 CART cells – progress but TME/antigen escape hurdles
- Procaspase 3: apoptosis revival?”

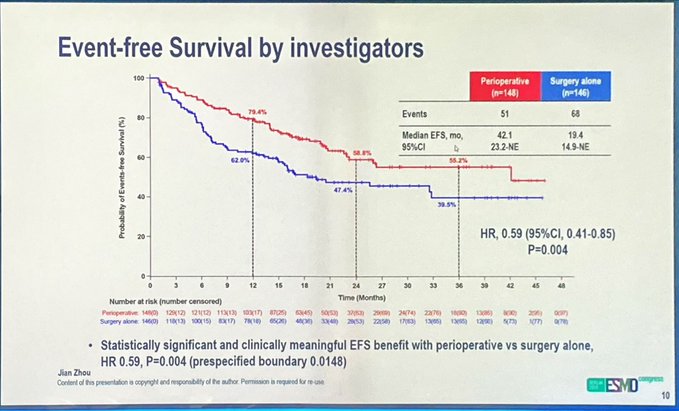
“CARES009 trial in HCC from ESMO.
Perioperative Camrelizumab+Rivoceranib vs surgery in resectable HCC (China)
- 93% completed neoadjuvant.
- 110 patients received adjuvant.
- Surgical complic similar.
EFS HR 0.63 (=BIRC).
Benefit across groups.
Major path resp 35.1%.”
“If you told me over a decade ago that bench-to-clinic research in cancer would see patients with SCLC potentially living for over 2 years.
Approximately 50% of patients with weight-normalized NSCLC survive 5 years.
AGA live 7 years.
I wouldn’t have believed you.
It’s all the more personal having a friend living with lung cancer.”
“ESMO25 has come to an end!
Loved seeing friends at Vall d’Hebron Institute of Oncology (VHIO) and colleagues from around the world.
Great discussions on:
- Early-drug development
- ctDNA
- ADCs
Inspired and grateful for the science and people. See you soon!”

“A rare win in a rare cancer:
Combining Delcath’s liver-targeted chemo (HEPZATO) with immunotherapy (ipi + nivo) helped 55% of patients stay cancer-free for a year vs 16% with chemo alone.
New hope for metastatic uveal melanoma.”
“ESMO Berlin is presenting groundbreaking research in melanoma treatment
For the first time, a ‘fixed’ mRNA vaccine – easier and cheaper to produce than personalized ones – has doubled the response rate in patients with advanced, treatment-resistant melanoma.
The vaccine BNT111 trains the immune system to attack cancer cells expressing four common melanoma proteins.
- In combination with immunotherapy (Cemiplimab), it achieved an 18% response rate, with 11.7% complete responses (CR) and 47.8% overall survival at 24 months.
- As a monotherapy, BNT111 reached a 17% response rate, including 13% complete responses (CR) and a 54.2% disease control rate (DCR) at 24 months.
- The treatment was well tolerated, with manageable side effects such as fever and chills.
A major step forward for mRNA technology – from fighting viruses to treating cancer.”
“Piecing Together The Patient Experience from Antibody-Drug Conjugates in Advanced Breast Cancer.
This satellite symposium at ESMO25 EONS18 discussed the experience of using ADCs within the context of Advanced Breast Cancer.
The discussion provided insights from a stellar panel of experts, Karin Jordan, Birgitte Grube, and Danijela Dohnal-Suvajac. We discussed ADC-induced Adverse Events, including Nausea and Vomiting and Fatigue, that are amongst the most frequent and can significantly affect the clinical outcomes as well as the patient’s quality of life.
Discussion also included severe adverse events (AE) such as ILD – Interstitial lung disease/pneumonia is which can have life-threatening consequences for the patient if not timely diagnosed and treated accordingly.
The pivotal role of the cancer nurse has been highlighted throughout the treatment with ADCs as follows:
- Establishing good communication with the patient and family and the MDT team.
- Supporting and educating the patient to increase the self-assessment of early symptoms that might indicate severe AEs, such as ILD.
- Early diagnosis of ADC-related AEs and appropriate referrals.
- Emotional support and psychosocial intervention.
Result: Improved Patient Outcomes
- Improved Symptom Control.
- Improved treatment adherence.
- Delayed progression and prolonged survival.
- Enhanced quality of life.”

“Post ESMO2025 EONS18, you always have a mix of feelings.
I’m tired after a very intense week, but I have my notebook full of ideas and inspiration, so while drained of energy, I have a lot to keep me going. The passion that the European Oncology Nursing Society (EONS) always transmits.
I met a lot of nice people,
I missed seeing many others that I would have loved to see,
I wanted to go to so many sessions that I would have needed 3 Celias to do so,
I was proud to see Annie Young recognized for her amazing career.
I have loved to hear a lot about prevention, and I got to learn about AIs by presenting with my amazing colleagues. I had the honor to present the From Testing to Targeted Treatments (FT3) and GPON joined initiatives with another group of Amazing speakers, also talking about precision medicine. I was impressed by many initiatives on storytelling, working under war circumstances, caregivers need…
I heard healthcare professionals advocating for good support care and the need to keep up with patients’ needs and questions. I had the luck to sit in a workshop with Young Cancer Europe.
I felt it was more interdisciplinary than ever.
Congratulations to the Scientific Committee. It was a difficult venue to navigate, but an amazing program to learn.
Congratulations, EONS teams, for always being there, helping and supporting everyone, and helping us to find the correct rooms.
Thank you!”

“ESMO – European Society for Medical Oncology Gastrointestinal Oncology Journal
Editorial board meeting together with my wonderful co-editor-in-chief, Irit Ben-Aharon, MD, PhD. And many of our outstanding editorial board members.
The journal is developing very well, being listed in Scopus, increasing submissions, publishing high-quality research articles, educational reviews, trials in progress, and provocative pieces of discussion.
Dynamic discussion among editorial board members – coming up with inspiring novel ideas.
Thank you all for joining!”

Union for International Cancer Control (UICC):
“UICC representatives Maribeth Walker, Marteen De Winter, CEO Dr Cary Adams, and Natasha Madsen Shah, Executive Director of the ATOM Coalition, were on the ground in Berlin for the ESMO – European Society for Medical Oncology (ESMO) Congress 2025.
ESMO25 offered a unique platform for collaboration, innovation, and the exchange of knowledge to shape the future of cancer care.
The UICC team had the opportunity to meet with partners, members, and peers – sparking new conversations and connections to advance global cancer control.
A big thank you to everyone we met in Berlin for the inspiring discussions and shared commitment to improving cancer care worldwide.”

“At ESMO, the presence of pathologists is becoming increasingly visible. From biomarker-driven trials to liquid biopsy, from digital pathology to AI-based prediction models, our work connects molecular discovery with real clinical impact.
t’s inspiring to see so many colleagues contributing to ESMO sessions, posters, and workshops, proving that precision oncology is not only about drugs, but also about data and interpretation.
Let’s keep making pathology matter in medical oncology!
And thank you to ESMO – European Society for Medical Oncology for recognizing the voice of pathology within the precision oncology community.”

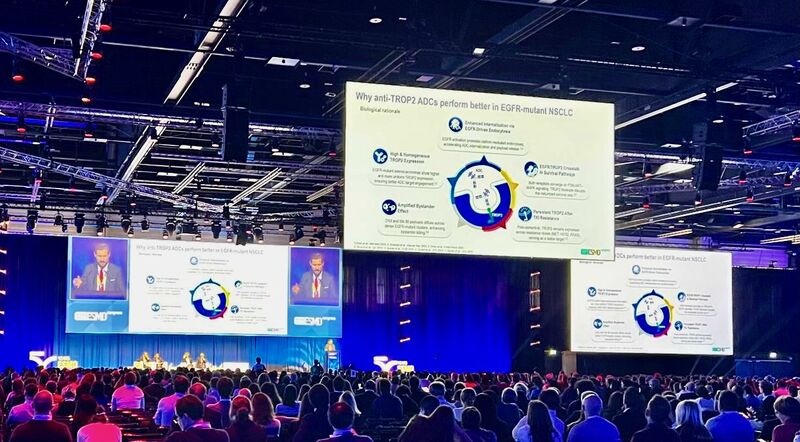
“EGFR-mutant lung cancer: the landscape is being rewritten.
During ESMO25, in front of more than 9,000 colleagues attending the Presidential Session II, I had the honor to serve as discussant for the pivotal phase 3 OptiTROP-Lung04 trial, presented by Prof. Li Zhang (Guangzhou, China).
This study represents a historic milestone for the EGFR-mutated non-small cell Lung Cancer community: the first phase 3 trial of a TROP2-directed ADC (Sac-TMT) to show statistically significant and clinically meaningful improvements in both PFS and OS versus platinum-based chemotherapy.
In my discussion, I focused on how the biological rationale and translational evidence supporting TROP2 targeting in EGFR-mutant disease are robust, innovative, and conceptually transformative.
At the same time, I emphasized the importance of further evaluating intracranial activity, a critical aspect for fully defining clinical benefit in this setting.
While MARIPOSA-2 currently defines the only globally approved standard of care, ongoing global phase 3 trials (e.g., TRO-Fuse009, Tropion-Lung15, Izabright-Lung-01, AndroMET-Lung-713) are already shaping the next frontier to optimize sequencing and extend benefit to patients worldwide.
The trajectory of EGFR mutant NSCLC is changing fast. With the evolution of first-line combinations (MARIPOSA and FLAURA2), our approach to disease control, resistance, and long-term outcomes is being redefined in real time.”
“Lung update:
HARMONi-6
In untreated advanced squamous NSCLC, Ivonescimab plus chemotherapy significantly improved progression-free survival over Tislelizumab plus chemotherapy, with consistent benefit across PD-L1 levels and manageable safety.
OptiTROP-Lung04
In EGFR-mutated advanced NSCLC that progressed after EGFR-TKI therapy, Sacituzumab Tirumotecan significantly improved progression-free and overall survival compared with platinum-based chemotherapy, with manageable toxicity.
DREAM3R(LBA104)
Durvalumab combined with pemetrexed and platinum chemotherapy increased response rates but showed similar survival to chemotherapy alone in advanced pleural mesothelioma, with consistent safety and no predictive role for PD-L1.
NORTHSTAR
In patients with EGFR mutant NSCLC, consolidation local radiotherapy added to Osimertinib improved PFS.
Breast update:
DESTINY-Breast05
In patients with high-risk breast cancer HER2 HER2-positive with residual disease after neoadjuvant therapy, T-DXd demonstrated superior efficacy with manageable safety, compared to TDM-1.
Destiny breast 09 (LBA18)
T-DXd plus Pertuzumab significantly improves outcomes versus THP across all subgroups of HER2-positive advanced or metastatic breast cancer, with a consistent safety profile.
DESTINY-Breast11
Trastuzumab deruxtecan (T-DXd) alone or followed by Paclitaxel plus Trastuzumab and Pertuzumab (T-DXd-THP) versus dose-dense Doxorubicin plus cyclophosphamide followed by THP (ddAC-THP) as neoadjuvant therapy for high-risk HER2-positive early breast cancer.
ASCENT-03
In patients with advanced triple-negative breast cancer ineligible for PD-1 or PD-L1 inhibitors, Sacituzumab Govitecan significantly improved progression-free survival and response durability compared to standard chemotherapy, with a similar safety profile.
GI update:
FOxTROT and NICHE-2 Comparison(724O)
Neoadjuvant Nivolumab/Ipilimumab achieved superior 3-year disease-free survival and pathological response compared to chemotherapy in locally advanced dMMR colon cancer, supporting its use as the preferred neoadjuvant approach.
CARES-009(1470O)
Perioperative Camrelizumab plus Rivoceranib significantly improved event-free survival and pathological response compared to surgery alone in resectable hepatocellular carcinoma at intermediate or high risk of recurrence.
GU Update:
SGNDV-001
Disitamab vedotin combined with Toripalimab significantly improves survival and response rates with a more favorable safety profile compared to chemotherapy in untreated HER2-expressing advanced urothelial cancer.
KEYNOTE-905/EV-303
In patients with muscle-invasive bladder cancer ineligible for cisplatin, perioperative Enfortumab vedotin (EV) plus Pembrolizumab (Pembro) versus surgery alone significantly improved event-free survival, overall survival pathological complete response rate.
PSMAddition(LBA6)
In PSMA-positive metastatic hormone-sensitive prostate cancer, adding [177 Lu]Lu-PSMA-617 to ADT plus ARPI significantly improved radiographic progression-free survival with manageable toxicity and no meaningful impact on quality of life.
CAPItello-281 Phase III study
In PTEN-deficient metastatic hormone-sensitive prostate cancer, adding the AKT inhibitor capivasertib to abiraterone and ADT significantly prolonged radiographic progression-free survival with manageable, expected toxicities.
EnzaRad(LBA86)
Adding enzalutamide to ADT and radiation provided a limited overall survival benefit in high-risk localized or locally advanced prostate cancer, with greater metastasis-free survival seen in patients with positive pelvic nodes or planned pelvic radiation.
EMBARK(LBA87)
Enzalutamide plus leuprolide significantly improves overall survival and delays disease progression compared with leuprolide alone in high-risk biochemically recurrent prostate cancer, supporting it as a standard-of-care option.”
“Bravo and congrats to my two very talented MD PhD students for having been awarded for the best ESMO fellowship project (Marcela Carausu Carausu) and for the best ESMO fellowship paper (Laetitia Collet Collet) at the ESMO25.”

“What an ESMO!
Reconnecting with friends and mentors, celebrating with colleagues, and having the honor of chairing a truly inspiring session with outstanding speakers – this year’s congress was a reminder of how energizing our oncology community can be.
Even a canceled flight and a 12-hour train ride couldn’t dampen the spirit -especially when shared with the best husband.
Thank you, ESMO, for the science, the inspiration, and the unforgettable moments. Until next time!”

You can also read ESMO 2025 Day 4 Highlights Not to Miss



