Vimseltinib (ROMVIMZA™) received approval from the European Commission (EC) on September 17, 2025, as the first and only therapy authorized in the European Union for adult patients with symptomatic tenosynovial giant cell tumor (TGCT) associated with functional decline, where surgery is no longer feasible or would result in unacceptable morbidity.

This milestone follows its U.S. Food and Drug Administration approval in 2024, making vimseltinib the first systemic treatment for TGCT on both sides of the Atlantic. With the EC’s decision, vimseltinib is now recognized as the only globally approved targeted therapy for TGCT.

“The European Commission’s approval of vimseltinib for TGCT is a significant milestone for Deciphera, ONO, and TGCT patients across the European Union who are in need of a non-invasive treatment option. We are excited to leverage our global commercial infrastructure to bring vimseltinib to these patients. We look forward to working with health authorities to ensure all eligible patients who can benefit from vimseltinib have access as quickly as possible.”
said Ryota Udagawa, President and Chief Executive Officer of Deciphera.

“This is welcome news for the TGCT community as vimseltinib is now the first approved therapy for TGCT in Europe. TGCT can significantly impact the daily lives of patients by causing pain, stiffness and mobility limitations. Vimseltinib is a differentiated treatment that has demonstrated the ability to address these unmet patient needs while remaining well-tolerated.”
said Jean-Yves Blay, M.D., Ph.D., Leon Berard Center.
What Drug is Vimseltinib (Romvimza)?
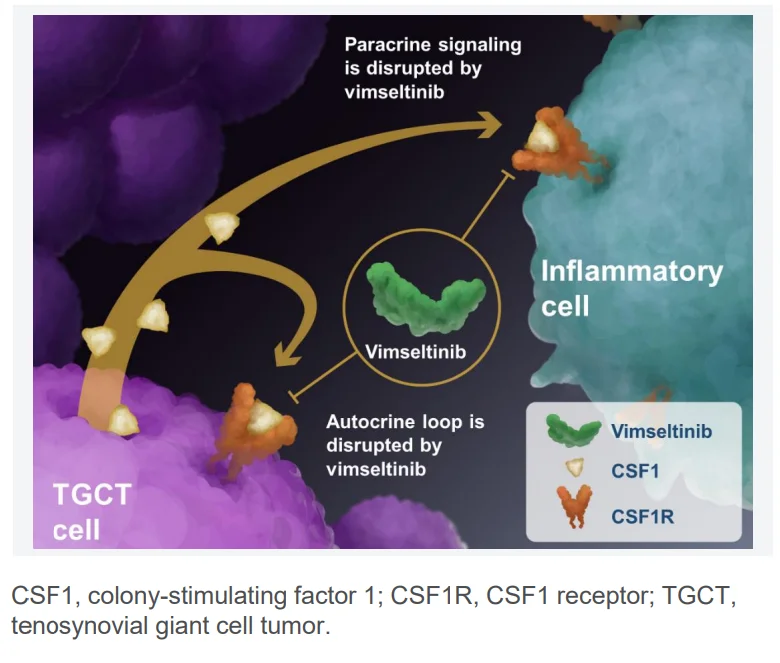
Vimseltinib (Romvimza) is an oral tyrosine kinase inhibitor (TKI) designed to selectively target the colony-stimulating factor 1 receptor (CSF1R). By blocking this receptor, the drug inhibits the proliferation of tumor-associated macrophages, which are central to the development and progression of tenosynovial giant cell tumor (TGCT).
Colony-stimulating factor 1 (CSF1), also known as macrophage colony-stimulating factor (M-CSF), is a cytokine that regulates the production, differentiation, and function of monocytes and macrophages. CSF1R signaling is essential for macrophage survival, differentiation, and growth. In TGCT, abnormal overproduction of CSF1 drives the excessive accumulation of macrophages, leading to tumor expansion, inflammation, and joint damage.
By inhibiting CSF1R, vimseltinib disrupts this pathological process, reducing macrophage-driven tumor growth, improving mobility, and relieving pain. Beyond TGCT, CSF1 and CSF1R pathways are also important in immune regulation, tissue repair, and homeostasis—functions that, when dysregulated, can contribute to cancer progression and other diseases.
Crash Course on Tenosynovial Giant Cell Tumor
Tenosynovial Giant Cell Tumor (TGCT) is a rare, typically non-malignant tumor that arises in the synovium, bursae, or tendon sheaths. It is driven by overexpression of colony-stimulating factor 1 (CSF1), which recruits and stimulates macrophage-like cells, leading to tumor formation and joint damage. TGCT is categorized into two main types:
- Localized TGCT (L-TGCT): Usually limited to a single joint or tendon sheath, most often seen in the fingers or knee.
- Diffuse TGCT (D-TGCT): A more aggressive form that affects larger joints such as the knee, hip, or ankle, and is associated with a higher risk of recurrence.
Patients with symptomatic TGCT often endure chronic pain, stiffness, swelling, and impaired joint function. Surgery has long been the primary treatment, but it carries risks and is frequently followed by recurrence, especially in diffuse disease. Until recently, systemic treatment options were virtually absent
About MOTION Trial
The MOTION trial (NCT05059262) was a multicentre, randomized, double-blind, placebo-controlled, phase 3 study. Patients aged ≥18 years with histologically confirmed, symptomatic TGCT not amenable to surgery were eligible. Key inclusion required lesions ≥2 cm confirmed by MRI and multidisciplinary assessment that surgery would cause severe morbidity or functional decline. Prior systemic anti-CSF1/CSF1R therapy was not allowed, though prior imatinib or nilotinib was permitted.
Patients were randomized 2:1 to oral vimseltinib 30 mg twice weekly or matching placebo, given in 28-day cycles for 24 weeks. Randomization was stratified by tumor location (lower limb vs other) and region (USA vs non-USA). At week 25, placebo patients could cross over to open-label vimseltinib.
The primary endpoint was objective response rate (ORR) at week 25, assessed by independent radiologic review using RECIST v1.1. Key secondary endpoints included tumor response by tumor volume score (TVS), range of motion, patient-reported physical function (PROMIS-PF), stiffness numeric rating scale, EuroQoL visual analogue scale (EQ-VAS), and Brief Pain Inventory (BPI) worst pain response. Safety was assessed using CTCAE v5.0.
Results
The MOTION trial showed that vimseltinib provided marked clinical benefit compared with placebo, with significant improvements in both tumor response and patient function. At the week 25 analysis, vimseltinib consistently outperformed placebo across radiographic endpoints, range of motion, and patient-reported outcomes.
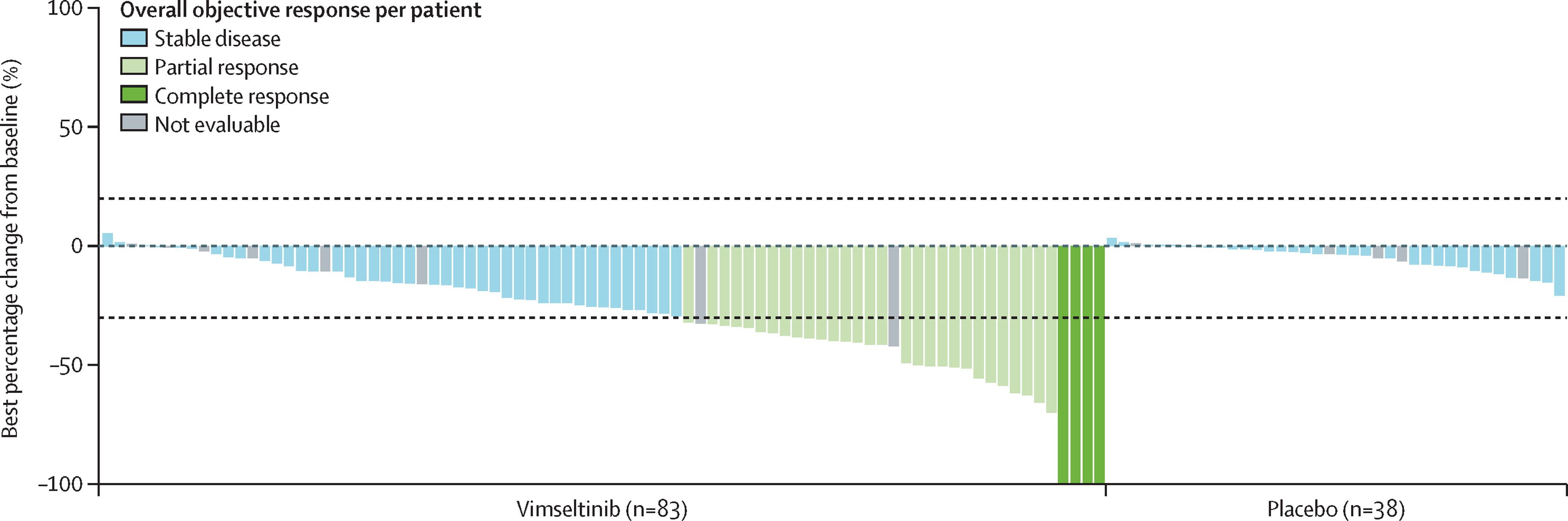
- Objective Response Rate (ORR) by RECIST: 40% (33 of 83) with vimseltinib versus 0% (0 of 40) with placebo (difference 40%, 95% CI 29–51; p<0·0001). This included 5% complete responses and 35% partial responses.
- ORR by Tumor Volume Score (TVS): 67% (56 of 83) with vimseltinib versus 0% with placebo (difference 67%, 95% CI 56–77; p<0·0001).
Functional outcomes:
- Range of motion: least squares mean improvement of +18.4% with vimseltinib versus +3.8% with placebo (difference 14.6 points; p=0.0077).
- PROMIS-PF: +4.6 versus +1.3 (difference 3.3; p=0.0007).
- Worst stiffness NRS: –2.1 versus –0.3 (difference –1.8; p<0.0001).
- EQ-VAS: +13.5 versus +6.1 (difference 7.4; p=0.016).
- BPI worst pain response: 48% versus 23% (difference 26%; p=0.0056).
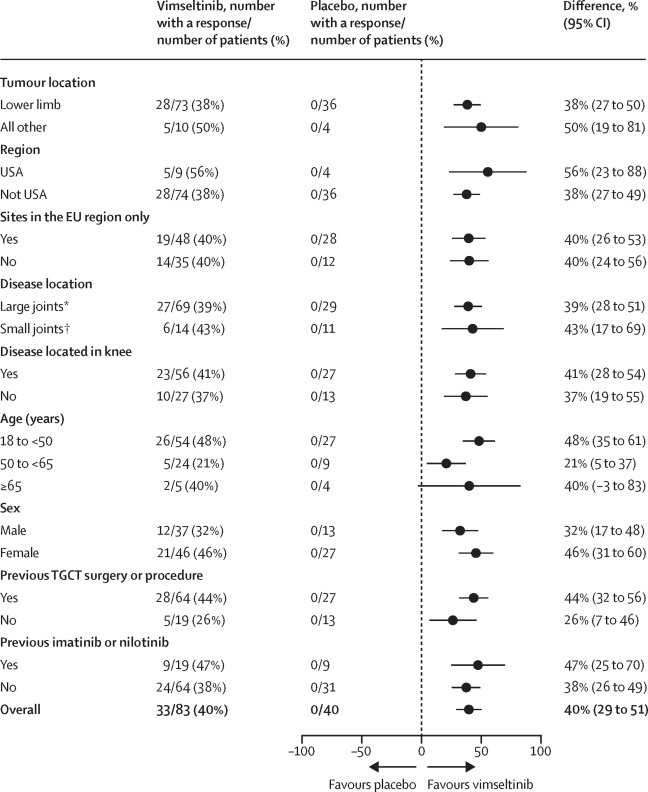
Notably, nearly all patients treated with vimseltinib (95%) experienced some degree of tumor shrinkage. Importantly, symptomatic improvements were reported even among patients classified as having stable disease by RECIST, highlighting the functional benefit beyond strict radiographic response criteria.
Safety
- Manageable safety profile; no liver toxicity observed
- Grade 3–4 TEAEs: 37% with vimseltinib vs 10% with placebo
- Only grade 3–4 AE >5%: elevated creatine phosphokinase (10%)
- Common AEs: periorbital edema (45%), fatigue (33%), pruritus (29%), facial edema (31%), headache (28%)
- Dose interruptions in 53% (short, median 4 doses missed); 70% remained on full dose
- Discontinuation rate low (6%)
Key Findings
- Vimseltinib achieved a 40% ORR by RECIST and 67% by TVS, markedly superior to placebo.
- Improvements extended beyond tumor shrinkage, with significant gains in range of motion, physical function, stiffness, pain, and health status.
- Median duration of response was not reached at data cutoff.
- The drug showed a manageable safety profile without hepatotoxicity, a major limitation of pexidartinib.
- Even patients with stable disease experienced meaningful symptomatic relief, suggesting vimseltinib reduces inflammatory symptoms as well as tumor mass.
A New Milestone in TGCT Treatment
The approval of vimseltinib marks an important milestone for patients with tenosynovial giant cell tumor, a rare but debilitating disease long limited to surgical management or a single systemic option with significant safety concerns.
By delivering robust tumor responses, improving mobility and symptoms, and avoiding hepatotoxicity, vimseltinib addresses a major unmet need for safer, effective systemic therapy. Its availability has the potential to transform care for this young, active patient population, offering meaningful relief and preserving quality of life where few options previously existed.
You can read the full article here.


