On February 14, 2025, the U.S. Food and Drug Administration (FDA) approved Vimseltinib (Romvimza) for adults with symptomatic tenosynovial giant cell tumor (TGCT) when surgical resection may cause severe morbidity or functional impairment. This approval marks a major milestone in providing a systemic treatment option for patients with TGCT, a rare but debilitating disease.
What is Vimseltinib (Romvimza)? Mechanism and How It Works
Vimseltinib (Romvimza) is an oral tyrosine kinase inhibitor (TKI) that selectively inhibits the CSF1 receptor (CSF1R), blocking the proliferation of tumor-associated macrophages, the key drivers of TGCT progression.
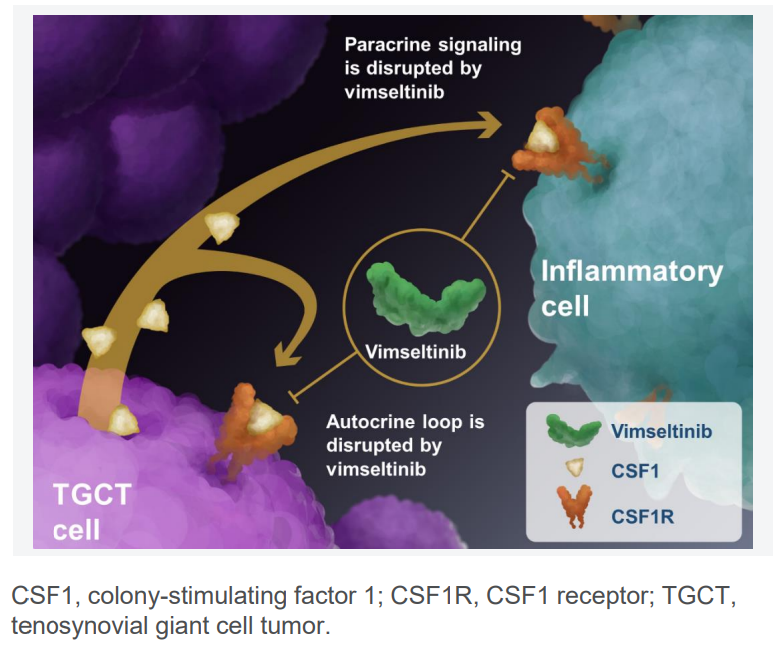
How Does CSF1R Inhibition Work?
Colony-stimulating factor, also known as macrophage colony-stimulating factor (M-CSF), is a cytokine that regulates the production, differentiation, and function of macrophages and monocytes
- CSF1R signaling plays a major role in macrophage growth, differentiation, and survival.
- In TGCT, excessive CSF1 production causes the abnormal accumulation of macrophages, leading to tumor growth and inflammation.
- By blocking CSF1R, Vimseltinib prevents tumor-associated macrophages from proliferating, thereby reducing tumor size, improving joint function, and alleviating pain.
It plays a key role in Immune system regulation, tissue repair, and homeostasis. In cancer and other diseases, these functions can contribute to disease progression.
What is a Tenosynovial Giant Cell Tumor?
Tenosynovial Giant Cell Tumor (TGCT) is a rare, typically benign tumor that affects the synovium, bursae, and tendon sheaths. It is driven by overexpression of colony-stimulating factor 1 (CSF1), leading to an abnormal proliferation of macrophage-like cells. TGCT is classified into two types:
- Localized TGCT (L-TGCT) – Usually affects a single joint or tendon sheath, commonly found in the fingers or knee.
- Diffuse TGCT (D-TGCT) – More aggressive, often involving large joints like the knee, hip, or ankle, and has a higher recurrence risk.
Patients with symptomatic TGCT often suffer from chronic pain, joint stiffness, swelling, and decreased physical function. Until now, systemic treatment options were limited, and surgery was the primary approach, despite its associated risks.
MOTION Trial: Clinical Data Supporting FDA Approval of Vimseltinib
The FDA’s approval of Vimseltinib was based on results from the MOTION trial (NCT05059262), a randomized, double-blind, placebo-controlled Phase 3 study evaluating its safety and efficacy in patients with symptomatic TGCT who were not candidates for surgery.
This is a double-blind, multicenter, placebo-controlled trial in patients with TGCT for whom surgical resection may cause worsening functional limitation or severe morbidity. The trial was conducted in 2 parts. The first part was a double-blinded phase where patients were randomized to receive either Vimseltinib or placebo. A total of 123 patients were randomized: 83 to the Vimseltinib arm and 40 to placebo. Vimseltinib was administered orally, 30mg twice weekly, and administered for 24 weeks. Part 2 is a long-term treatment phase in which all participants receive open-label Vimseltinib.
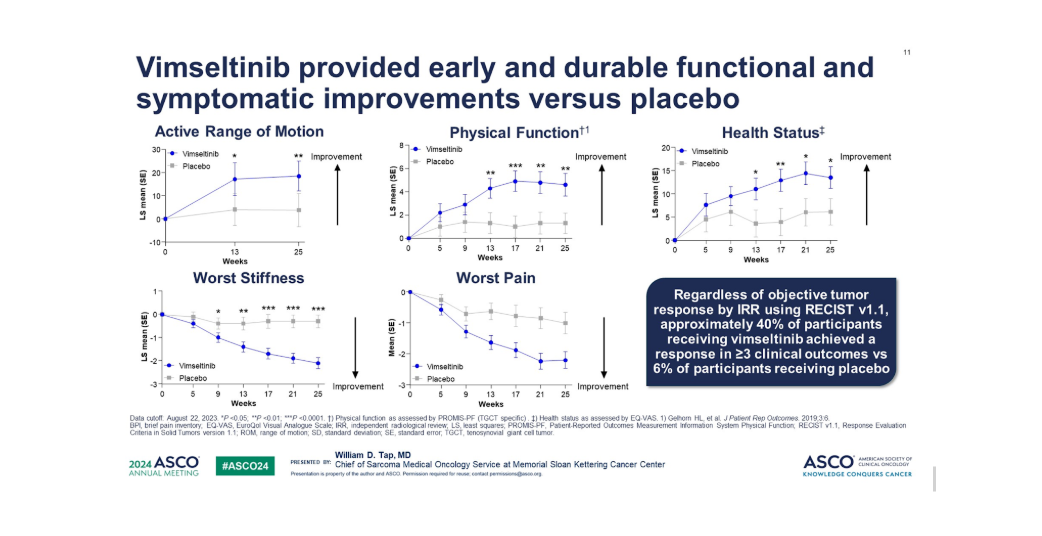
Efficacy Results of MOTION-3 Trial
Median duration of response (DOR) was not reached, but at 6 months, 85% had FOR ≥6 months, and 58% had DOR ≥9 months. Vimseltinib also showed significant improvements in range of motion, physical function, and pain compared to placebo.
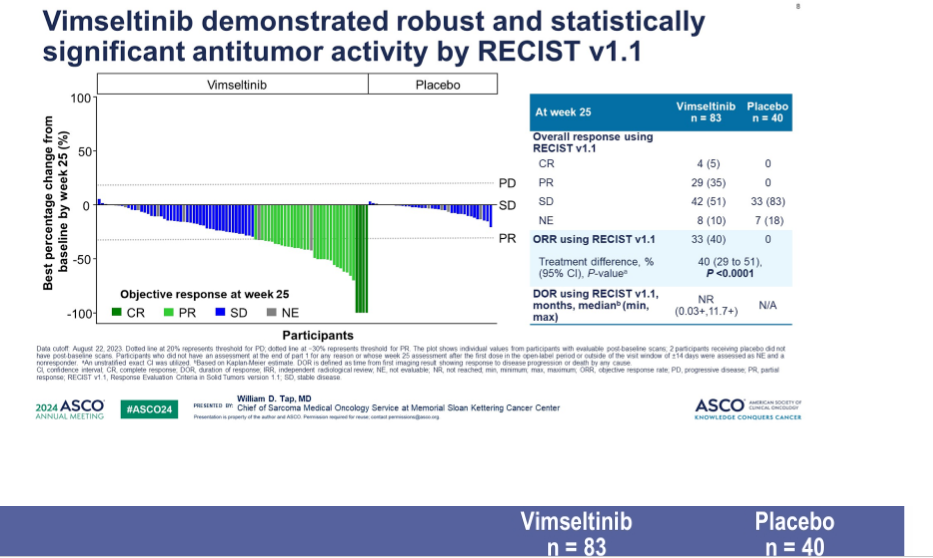
- Primary Endpoint: Overall Response Rate (ORR) at 25 weeks
- Vimseltinib: 40% ORR (95% CI: 29%-51%)
- Placebo: 0% ORR (95% CI: 0%-9%)
- p-value: < 0.0001 (highly significant)
- Median Duration of Response (DOR):
- 85% of responders had DOR ≥6 months
- 58% had DOR ≥9 months
The 1-year follow-up data from the MOTION 3 trial was presented at ESMO 2024 by Prof. Hans Gelderblom (Leiden University) and at the ASCO 2024 Annual Meeting by Dr. William Tap, Chief of Sarcoma Medical Oncology at MSK.
Vimseltinib Side Effects: What Patients Need to Know
Common adverse reactions (≥20%) included elevated AST, periorbital edema, fatigue, rash, high cholesterol, peripheral and facial edema, neutropenia, leukopenia, pruritus, and elevated ALT. The recommended Vimseltinib dose is 30 mg orally twice weekly, with at least 72 hours between doses.
Vimseltinib Common Side Effects (≥20%)
- Liver enzyme elevations (AST, ALT)
- Periorbital edema (eye swelling)
- Fatigue
- Rash
- Hypercholesterolemia (high cholesterol)
- Peripheral and facial edema
- Neutropenia, leukopenia (low white blood cell count)
- Pruritus (itching)
Vimseltinib Recommended Dosage and Administration
The recommended Vimseltinib dose is 30 mg orally twice weekly, with a minimum of 72 hours between doses.
A New Era for TGCT Treatment?
The FDA approval of Vimseltinib (Romvimza) represents a breakthrough in TGCT treatment, offering the first targeted systemic therapy for patients at risk of severe functional impairment. The MOTION trial data confirmed Vimseltinib’s efficacy in tumor reduction, pain relief, and functional improvement, filling a critical gap in treatment options.
With further clinical trials underway, Vimseltinib may pave the way for more advanced therapeutic strategies in TGCT management, improving outcomes for patients worldwide.
Written by Sona Karamyan, MD


