Botensilimab and balstilimab represent a novel immunotherapy strategy designed to enhance antitumor immune responses in immunologically “cold” solid tumors that are intrinsically resistant to conventional immune checkpoint blockade, including microsatellite-stable (MSS) metastatic colorectal cancer (mCRC).
In a phase I study (NCT03860272), this Fc-enhanced anti–CTLA-4 antibody combined with an anti–PD-1 agent demonstrated encouraging activity in heavily pretreated MSS mCRC. Given the modest survival benefits historically achieved with established late-line options such as regorafenib, trifluridine–tipiracil, fruquintinib, and trifluridine–tipiracil plus bevacizumab.
A cross-trial comparative analysis was performed using reconstructed individual patient survival data to contextualize the efficacy and safety of botensilimab plus balstilimab relative to current standards of care in the refractory mCRC setting.
Background
Microsatellite-stable (MSS) metastatic colorectal cancer (mCRC) remains one of the most immunotherapy-resistant “cold” tumor settings, and the refractory-line treatment landscape has historically been defined by modest survival gains with small-molecule multikinase inhibitors and late-line cytotoxics. For unselected patients who have progressed after fluoropyrimidine, oxaliplatin, and irinotecan (with anti-VEGF and, when appropriate, anti-EGFR therapy), established options have included regorafenib and trifluridine–tipiracil (FTD-TPI), with incremental advances more recently through fruquintinib and the SUNLIGHT regimen (FTD-TPI plus bevacizumab).
Against this backdrop, the phase I experience of botensilimab (Fc-enhanced anti-CTLA-4) plus balstilimab (anti–PD-1) reported unexpectedly prolonged survival signals in heavily pretreated MSS mCRC, prompting interest in how this immunotherapy doublet might compare with contemporary standards. The article by Bengala and colleagues addresses this question through an exploratory, hypothesis-generating cross-trial comparison using reconstructed individual patient time-to-event data from Kaplan–Meier curves across key refractory mCRC trials, contrasting efficacy and toxicity patterns between botensilimab + balstilimab and accepted late-line regimens.
Methods
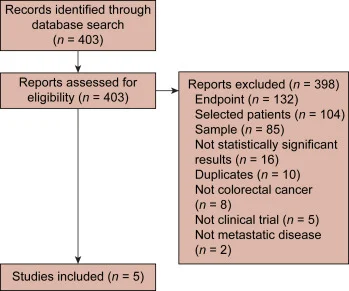
The investigators conducted a systematic PubMed search to identify clinically relevant trials in unselected refractory mCRC that reported Kaplan–Meier plots for overall survival (OS) and progression-free survival (PFS). Trials that selected patients by biomarker (mutations), metastatic site, or ethnicity were excluded, as were studies enrolling fewer than 100 patients. Because the intent was to compare regimens that shaped clinical standards, studies with negative or non–practice-changing outcomes were also excluded. Four pivotal phase III studies were selected as comparators—SUNLIGHT, FRESCO-2, RECOURSE, and CORRECT—and were compared with the botensilimab + balstilimab MSS mCRC cohort from NCT03860272 (phase I).
To enable cross-trial comparisons beyond summary medians, the authors reconstructed individual patient data (IPD) for OS and PFS from published Kaplan–Meier curves using a graphical algorithm. Hazard ratios (HRs) with 95% confidence intervals (CIs) were then derived. Safety comparisons focused on the most frequent treatment-related adverse events (TRAEs), extracting all-grade and grade 3–4 rates when available and summarizing differences using odds ratios (ORs) with 95% CIs.
Study Design
This work is best understood as an exploratory comparative effectiveness analysis, not a randomized head-to-head study. The included trials differed by design, era, eligibility criteria, and line of therapy distribution:
- Botensilimab + balstilimab (NCT03860272): phase I, open-label, safety primary endpoint; 148 MSS mCRC patients treated with botensilimab 1 or 2 mg/kg every 6 weeks plus balstilimab 3 mg/kg every 2 weeks. Response evaluability was defined in the original report by follow-up requirements; the article notes 101 patients had ≥6 months follow-up and were evaluable for response.
- SUNLIGHT (phase III): 492 patients, 1:1 randomization; FTD-TPI + bevacizumab (n=246) vs FTD-TPI alone (n=246); OS primary endpoint.
- FRESCO-2 (phase III): 691 patients, 2:1 randomization; fruquintinib (n=461) vs placebo (n=230).
- RECOURSE (phase III): 798 patients; FTD-TPI (n=533) vs placebo (n=265).
- CORRECT (phase III): 760 patients; regorafenib (n=505) vs placebo (n=255).
The authors explicitly acknowledge potential bias introduced by differences in pretreatment intensity: the median number of prior lines was three across most trials, but two in SUNLIGHT, implying a less heavily pretreated population that could inflate OS/PFS relative to later-line cohorts. Patient-level covariate adjustment was limited because covariates were available only in aggregate form after reconstruction. The analysis is framed as hypothesis-generating, with the primary objective being OS comparison and secondary objectives including PFS and safety profile contrasts.
Results
Cross-trial survival comparisons
1) Botensilimab + balstilimab vs SUNLIGHT regimen (FTD-TPI + bevacizumab)
Immunotherapy was associated with longer OS, with an HR 0.62 (95% CI 0.44–0.89) favoring botensilimab + balstilimab over FTD-TPI + bevacizumab in reconstructed comparisons.
In contrast, the PFS comparison favored FTD-TPI + bevacizumab, with an HR 0.46 (95% CI 0.35–0.61) in favor of the SUNLIGHT regimen. The paper interprets early crossing of curves as suggestive of primary resistance in a subset receiving immunotherapy, with a potential delayed benefit emerging over time in responders—an important pattern in immunotherapy biology that can yield OS separation even when early PFS is not improved.

2) Botensilimab + balstilimab vs later-line standards (fruquintinib, regorafenib, FTD-TPI alone)
OS comparisons all favored immunotherapy, with statistically significant differences reported:
- Versus fruquintinib: HR 0.38 (95% CI 0.27–0.54)
- Versus regorafenib: HR 0.32 (95% CI 0.22–0.46)
- Versus FTD-TPI: HR 0.37 (95% CI 0.27–0.52)
PFS comparisons also generally favored immunotherapy:
- Versus regorafenib: HR 0.59 (95% CI 0.44–0.77)
- Versus FTD-TPI: HR 0.63 (95% CI 0.51–0.84)
Versus fruquintinib: no statistically significant difference, with HR 0.78 (95% CI 0.61–1.0) (trend but borderline).

Absolute outcomes highlighted from the botensilimab + balstilimab phase I experience
The manuscript anchors its comparisons in the reported phase I MSS mCRC outcomes: in the response-evaluable population, median PFS was 3.5 months and median OS was 20.9 months. These values stand out against historical late-line benchmarks (typically single-digit median OS in placebo-controlled phase III programs), which is why the authors emphasize the need for confirmatory randomized trials while still acknowledging the signal as clinically provocative.
Safety and toxicity profile differences
A central contribution of the paper is the toxicity pattern contrast between checkpoint blockade and established refractory regimens, which can shape treatment choice even before definitive comparative efficacy is confirmed.
For botensilimab + balstilimab, the most frequent TRAEs in the cross-trial table were fatigue (35%) and diarrhea (32%). Grade 3–4 rates were relatively low for fatigue (1%) and higher for diarrhea (5%), with significant differences depending on the comparator.
Compared with FTD-TPI + bevacizumab, immunotherapy had higher odds of fatigue (OR 1.97, 95% CI 1.25–3.11) and diarrhea (OR 3.78, 95% CI 2.40–5.94), including a notable difference for grade 3–4 diarrhea (OR 6.97, 95% CI 1.46–33.29).
Compared with cytotoxic/TKI options, immunotherapy showed less hematologic toxicity signals in the extracted comparisons. For example, anemia rates were lower with immunotherapy than with SUNLIGHT and markedly lower than with FTD-TPI monotherapy in RECOURSE-derived data (where anemia and neutropenia are well-known limiting toxicities in heavily pretreated patients).
The comparator trial summaries reinforce these differences: SUNLIGHT reported common AEs including neutropenia (62%), nausea (37%), and anemia (29%); FRESCO-2 emphasized hypertension (37%) with 14% grade ≥3; CORRECT highlighted high-grade hand-foot skin reaction (17%), fatigue (10%), diarrhea (8%), hypertension (7%), and rash/desquamation (6%).
Beyond rates, the article underscores management nuances in immune-mediated GI toxicity. It notes that infliximab was used even in low-grade settings for suspected immune-mediated diarrhea/colitis, with 36 patients receiving immunosuppressants, 35 receiving infliximab, and 31 receiving concurrent steroids; complete resolution occurred in 31 patients, supporting early proactive intervention as a feasibility signal for broader use if efficacy is confirmed.
Key findings
In reconstructed cross-trial comparisons, botensilimab + balstilimab was associated with improved OS versus multiple refractory mCRC standards, including an OS advantage versus FTD-TPI + bevacizumab (HR 0.62, 95% CI 0.44–0.89) and stronger OS advantages versus fruquintinib, regorafenib, and FTD-TPI (HR range 0.32–0.38).
PFS did not uniformly favor immunotherapy, with FTD-TPI + bevacizumab outperforming botensilimab + balstilimab for PFS (HR 0.46 in favor of SUNLIGHT), suggesting early progression in a subset and potential delayed benefit in responders.
The toxicity profile is qualitatively different: immunotherapy is characterized by fatigue/diarrhea and immune-mediated GI events, whereas chemotherapy/TKIs are dominated by hematologic toxicity (FTD-TPI), hypertension (anti-VEGF/TKIs), and dermatologic/hand-foot toxicity (regorafenib).
The analysis highlights the need for biomarker-informed selection, including clinical features such as liver metastasis status, based on exploratory data suggesting better outcomes in patients without active liver metastases.
Conclusion
Through a systematic selection of pivotal refractory mCRC trials and reconstruction of individual patient survival data from Kaplan–Meier curves, an exploratory comparison is provided suggesting that botensilimab + balstilimab may confer an OS advantage over contemporary standards, including SUNLIGHT and later-line regimens such as fruquintinib, regorafenib, and FTD-TPI monotherapy.
Definitive positioning of botensilimab + balstilimab therefore depends on ongoing randomized evaluation, including trials designed to identify predictive clinical and molecular biomarkers that can distinguish early resistance from durable benefit and optimize treatment selection in MSS mCRC.
You can read the full article here.