Talha Badar, Specialist in Hematology Oncology based in Mayo Clinic, shared on X:
“Will discuss DDX41mt most unique and most prevalent germline predisposition syndrome in myeloid neoplasms.
Epidemiological data, clonal evolution, variant classification, risk stratification, clinical presentation, and management in a thread.
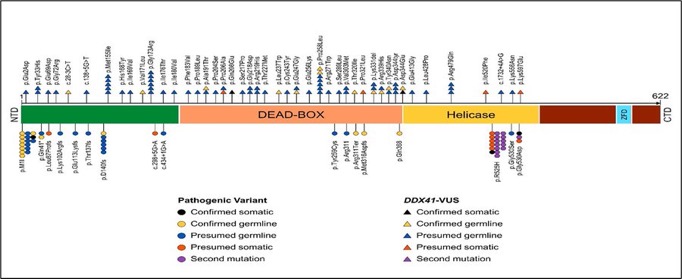
DDX41mt in MN
Salient features
- DDX41 (DEAD/H-box helicase) gene located on chromosome 5q35.3mutation (m) GPS; most prevalent GPS associated with MN(2%-5%).
- Male predominant, presents at advance age, without familial clustering.
- Assoc. with hypocellular BM, runs indolent course.
- Assoc. with normal CG, conventional prognostic factor do not adequately stratify.
Prevalence
Germline and somatic targeted sequencing was performed in adult pts with hypoplastic BM to study GPS variants and their clinical correlates.
27/402 (6.7%) patients, carried germline variants.
Most common in order; were DDX41, Fanconi anemia, GATA2, severe congenital neutropenia, RASopathy, and Diamond-Blackfan anemia.
Molteni et al. Blood 2023
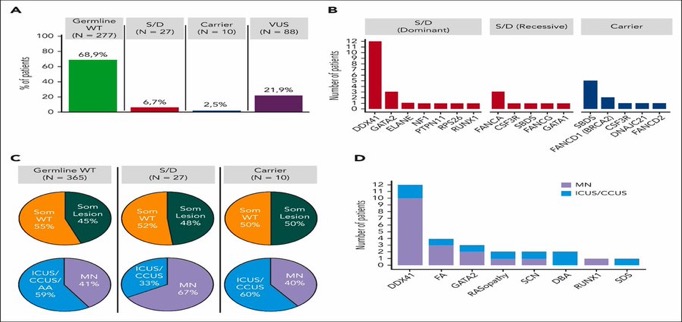
Clonal evolution, somatic mosaicism:
Somatic mutations in the unmutated DDX41 allele (e.g.R525H) as a common secondary alteration seen in 39-50% of patients resulting in leukemic transformation.
ARCH: DNMT3A, ASXL1, TET2, and TP53, rates are lower compared to other GPS.
This atypical pattern suggest distinct myeloid disease progression.
Homan et al. Blood Adv 2023 and our abstract at ASH2023
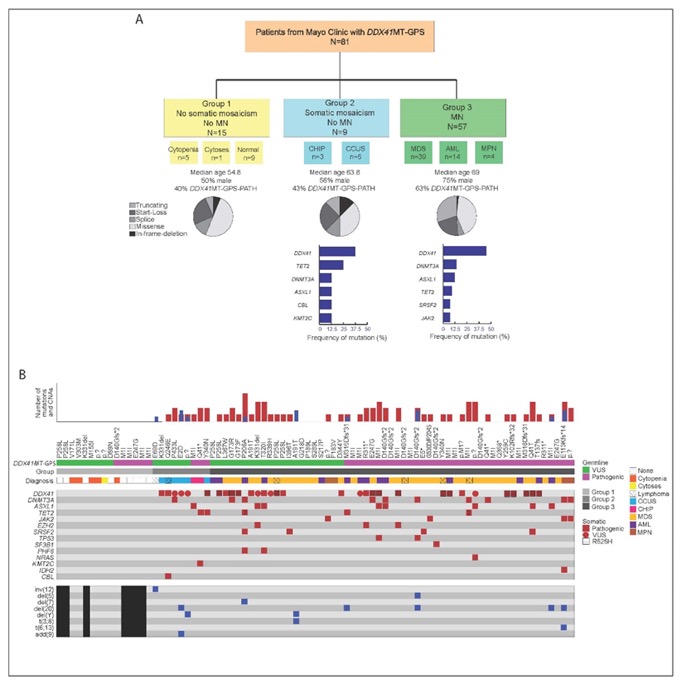
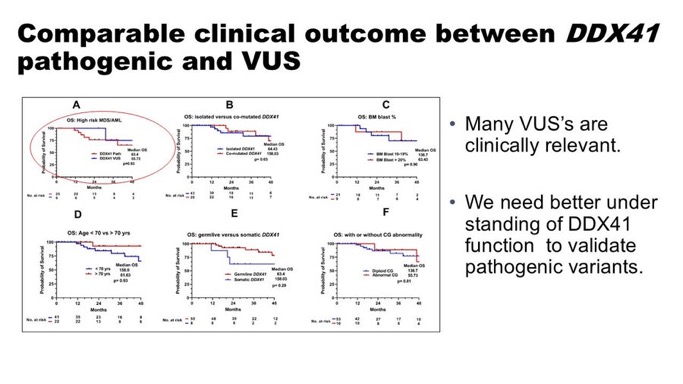
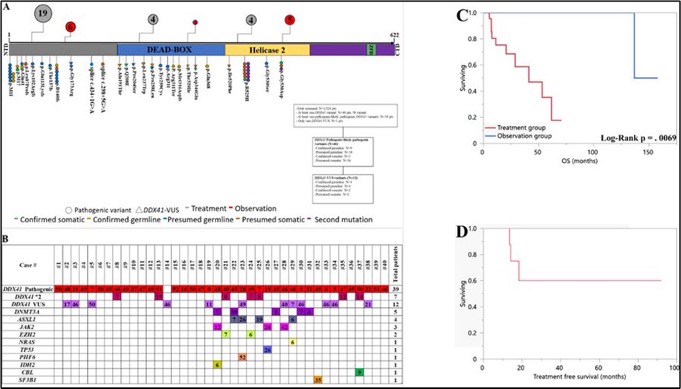
DDX41mt variant classification: pathogenic vs likely pathogenic vs VUS
• Variant classification is challenging, with conflicting classifications.
• Common variants: M1I/D140fs/G173R (pathogenic), P258L/R479Q/M155I/R339H (VUS).
Utilizing ACMG classification, we observed comparable outcome between path and VUS
Our study with detailed analysis
Thanks to Mrinal Patnaik for supervision and Alex Ferrer for guidance with variant curation.
Clinical phenotype:
Unlike other GPS, presents at advance age without familial clustering.
Does not manifest a proliferative phenotype, assoc. with hypocellular marrow, runs indolent course.
Do not usually accompany with adverse risk mutation or CG profile.
References: Alkhateeb et al. Blood Adv 2022, Badar and Chlon. Curr Hematol Malig Rep 2022, Makishima et al. Blood 2023
Management:
Several groups have reported favorable outcome of DDX41m MN, there are no specific guideline how to treat.
Several groups including ours have reported, ↑ response rate with lenalidomide in HR MDS and Venetoclax+HMA in AML.
However, which therapy works best for DDX41m AML is undetermined
References: Abou Dalleet al. AJH 2020, Nanaa and Dr. Alkali et al. BJH 2023
Role of allo-HCT in DDX41mt HR MDS and AML?
Considering DDX41m MDS/AML having favorable outcome. It is valid question to ask if we should consider these pts for allo-HCT in 1st response.
Our gp at Mayo Clinic Comprehensive Cancer Center conducted analysis among MDS & AML pts with DDX41m (Baranwal and Alkhateeb et al. BMT 2022)
Allo-HCT did not improve OS, associated with higher TRM.
Warrant prospective evaluation regarding role of alloHCT.
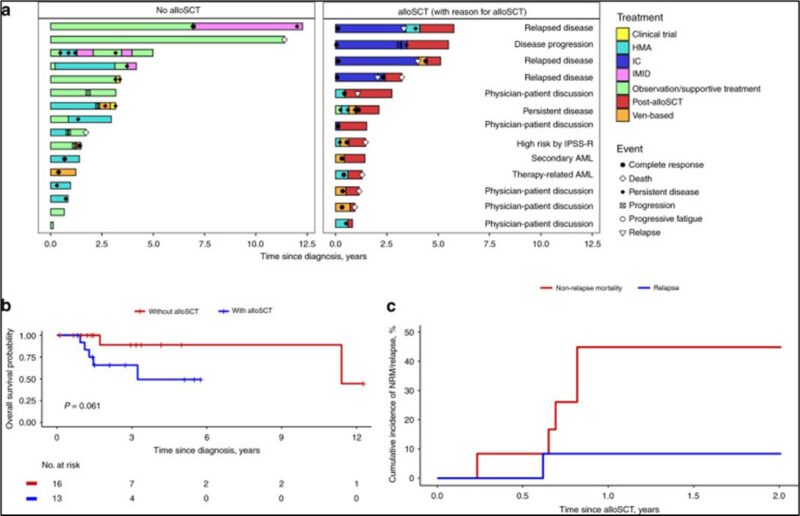
Can we observe elderly frail patients in late 70’s and 80’s?
Dr. Alkali led this study reporting outcome 40 DDX41mt HR MDS/AML pts.
27 (68%) pts received Rx with median time-to-treatment of 0.7 (0–3.2) mo, while 13 (32%) were observed; of which 9 (75%) received Rx later-on with median time-to-treatment of 16 (7.2–92) mo (none of the 13 pt died while under observation).
5-year OS of 100% in observation gp compared to median OS of 41 mo in Rx gp (p = 0.0069)
Summary:
1. DDX41mt is most prevalent in GPS assoc. wtih myeloid malignancies.
2. Most common somatic mutation R525H, CH is less common.
3. Variant classification is challenging, functional analysis are needed to determine path vs VUS.
4. Elderly, frail pts with low disease burden can be observe with close surveillance.
5. Usual risk stratification method are not accurate in DDX41m HR-MDS and AML.
6. Excellent responses to venetoclax +HMA.”
Dr. Talha Badar, MD, is a specialist in Hematology Oncology based in Mayo Clinic, Jacksonville, Florida. His primary areas of expertise include Leukemia, particularly Acute Myeloid Leukemia (AML), Myelodysplastic Syndrome (MDS), Acute Lymphoblastic Leukemia (ALL), and Bone Marrow Transplantation. Over his career, he has actively contributed to clinical research and clinical trials.


