Subcutaneous immunotherapy (SCIT), traditionally used to treat allergic conditions through repeated administration of specific antigens, is now being repurposed in oncology as a novel route for immune activation against cancer. While systemic and intratumoral immunotherapies have dominated the field, SCIT offers a unique mode of immune modulation—targeting dermal antigen-presenting cells to induce durable, tumor-specific T cell responses.
As the search for more tolerable, effective, and accessible cancer immunotherapies continues, SCIT has emerged as a promising strategy, particularly in cancer vaccine delivery and cytokine-based immune stimulation. This article reviews the scientific basis of SCIT in oncology, outlines key clinical and translational advances, and discusses its potential to complement and enhance existing immunotherapeutic modalities.
Repurposing Subcutaneous Immunotherapy for Cancer
Subcutaneous immunotherapy (SCIT) has been a proven strategy in allergology, effectively inducing long-term tolerance to allergens like pollen and venom through repeated subcutaneous injections. Its mechanism—modulating dendritic cells and generating antigen-specific regulatory T cells—has made it an appealing model for cancer immunotherapy.
In oncology, SCIT is now being reimagined not to induce tolerance but to stimulate robust anti-tumor immune responses. By delivering tumor-associated antigens or neoantigens subcutaneously, often with adjuvants like GM-CSF, researchers aim to enhance T cell priming in a controlled, localized manner.
SCIT’s use in cancer began with early peptide vaccine trials in the 1990s. Over time, its role expanded with the development of personalized neoantigen vaccines and subcutaneous cytokine delivery. Today, SCIT is emerging as a practical and immune-potent route to support cancer vaccines, immune modulators, and combination therapies—bridging lessons from allergy to oncology.
Mechanisms of Action in Cancer: The Immunologic Basis of Subcutaneous Delivery
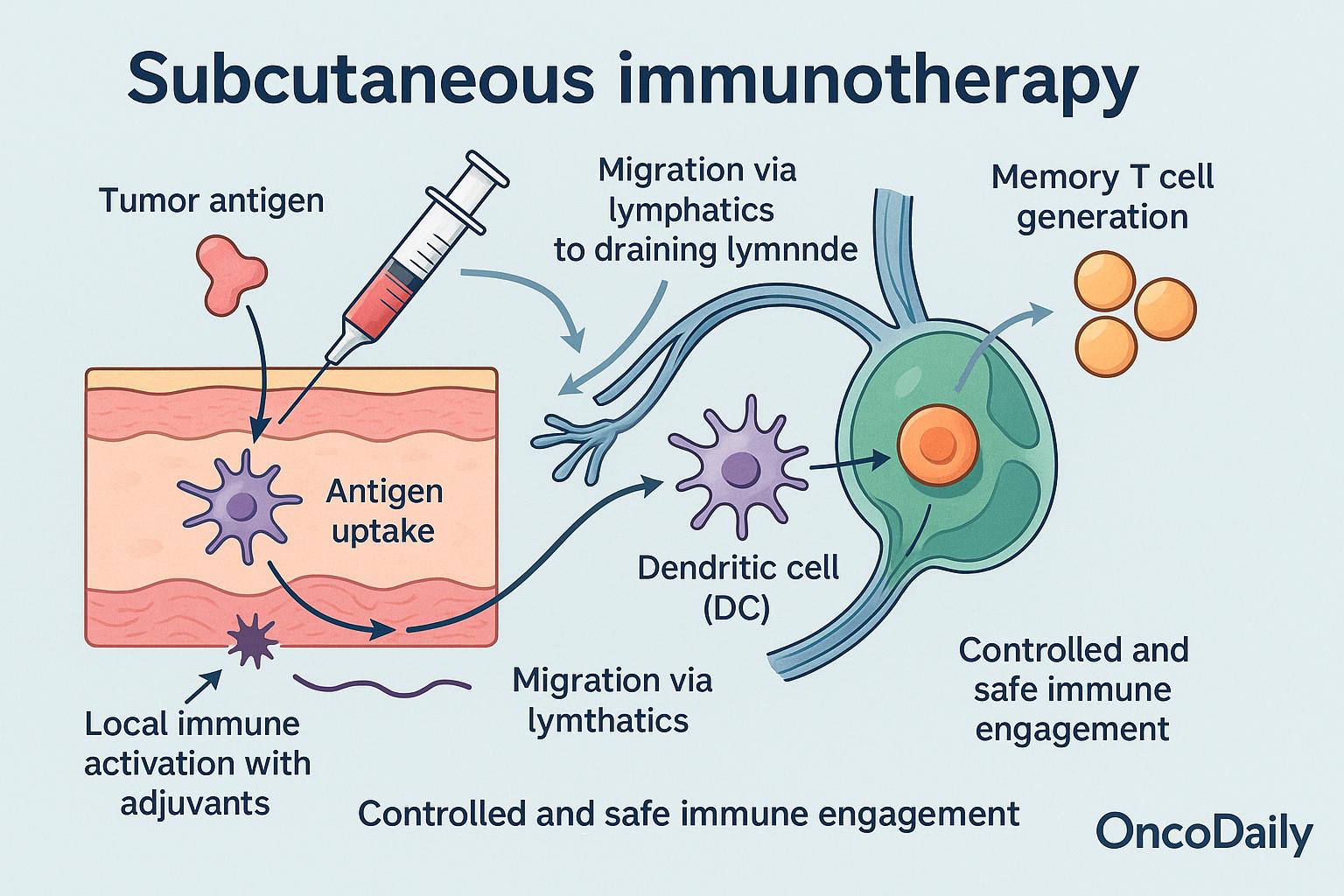
Subcutaneous immunotherapy activates the immune system through a series of well-orchestrated steps:
-
Antigen uptake by dermal dendritic cells (DCs): Tumor antigens delivered subcutaneously are rapidly captured by skin-resident dendritic cells.
-
Migration via lymphatics to draining lymph nodes: Activated DCs travel through lymphatic vessels to regional lymph nodes, where they present antigens to naïve T cells.
-
T cell priming and cross-presentation: Within the lymph nodes, dendritic cells cross-present tumor-associated antigens to CD8+ T cells, promoting cytotoxic T cell activation.
-
Memory T cell generation: In addition to immediate effector cells, this process supports long-term memory T cell formation, essential for sustained tumor control.
-
Local immune activation with adjuvants: Co-administered agents like GM-CSF, CpG (TLR9 agonist), or other TLR agonists enhance dendritic cell activation and amplify the immune response at the injection site.
-
Controlled and safe immune engagement: Unlike intravenous (IV) therapies, SCIT offers lower systemic exposure and reduced toxicity. Compared to intratumoral (IT) routes, it doesn’t require direct access to tumors and is easier to administer.
This mechanism supports SCIT as a powerful platform for cancer immunotherapy—capable of initiating strong, targeted immune responses with improved safety and accessibility.
Cancer Applications of Subcutaneous Immunotherapy
Subcutaneous immunotherapy is emerging as a powerful vehicle for cancer vaccines. In melanoma, Moderna’s personalized neoantigen mRNA‑4157 vaccine, delivered SC alongside pembrolizumab, significantly reduced recurrence risk by 49% and increased 2.5‑year recurrence-free survival from ~56% to ~75%. Early NSCLC studies of the same platform show encouraging safety and immune activation
The SC route is also enhancing cellular immunotherapies. Studies in renal cell carcinoma have shown that subcutaneous IL‑2, when used post-surgery or with CAR-T/TIL therapy, achieves ~17–20% response rates, including complete responses in ~3% of patients, with improved tolerability vs. IV high-dose regimens
Subcutaneous delivery is further being tested as an adjunct to checkpoint blockade. In melanoma and RCC, trials combining SC cytokines or TLR agonist adjuvants with anti‑PD‑1 demonstrate enhanced T cell priming and reshaping of the tumor microenvironment (e.g., EVX‑01 plus anti‑PD‑1).
- Melanoma (KEYNOTE‑942): mRNA‑4157 SC + pembrolizumab reduced recurrence/mortality by 49% at 3 years.
-
Resected RCC: SC IL‑2 in high‑risk patients yielded a 3‑year disease‑free survival of ~33% and overall survival of ~70%, with good tolerabilit.
Advantages and Limitations of Subcutaneous Immunotherapy in Cancer
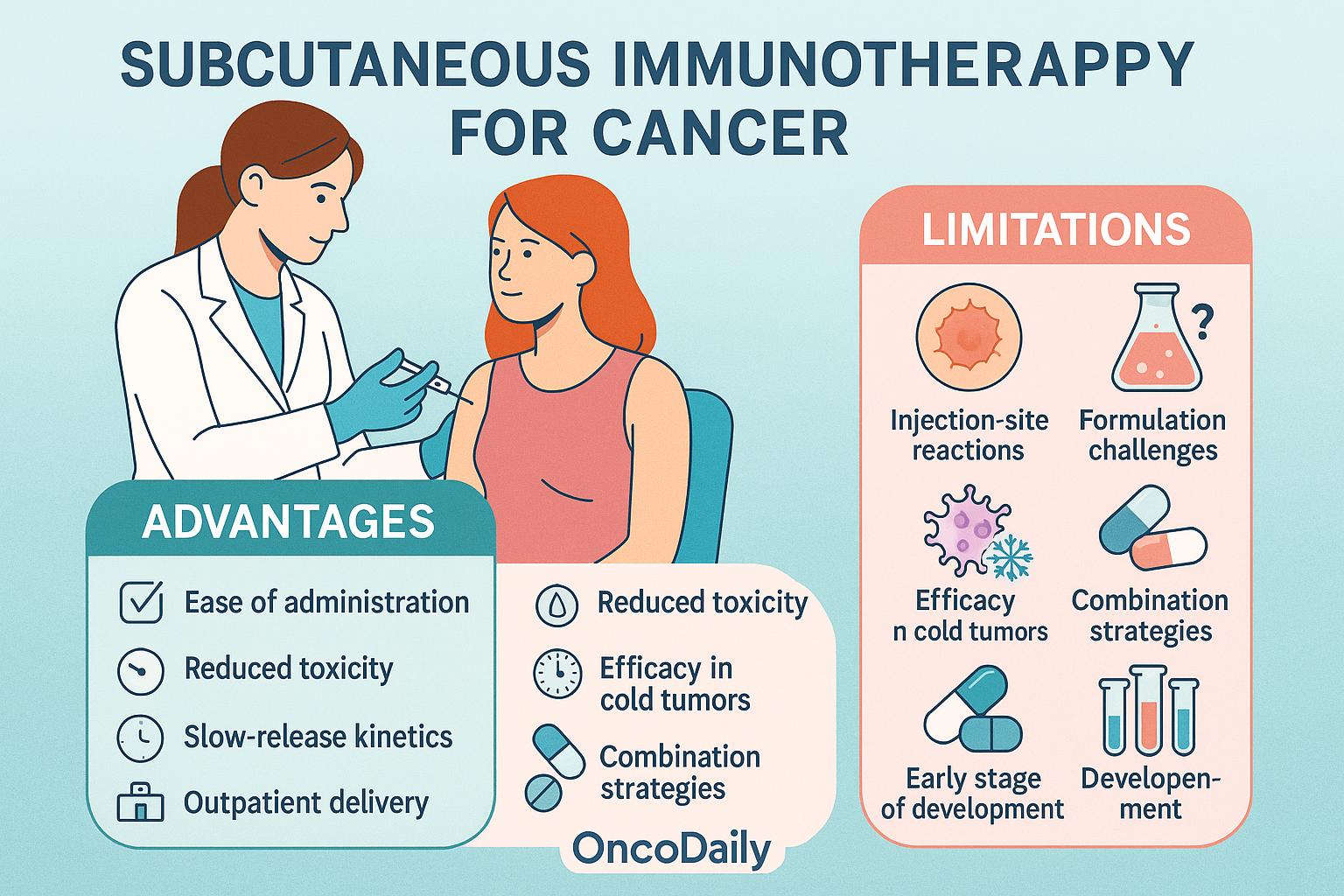
Subcutaneous immunotherapy (SCIT) provides several key advantages that make it attractive for cancer treatment. Its ease of administration in outpatient settings improves patient comfort and adherence, especially compared to intravenous (IV) therapies. The lower systemic exposure associated with SC delivery helps reduce toxicity—particularly for cytokines like IL-2—making it a safer alternative to high-dose systemic infusions. Additionally, the slow-release kinetics at the injection site enhance antigen presentation over time, supporting stronger and more durable immune responses.
Despite these benefits, SCIT also presents some limitations. Local injection-site reactions such as swelling or redness are common. There are also formulation and dosing challenges, especially when delivering large or complex molecules. As a monotherapy, SCIT may show limited efficacy in “cold” tumors lacking baseline immune infiltration, often requiring combination with other immunotherapies. Importantly, SCIT is still in the early stages of oncology development, with most applications currently under investigation in clinical trials and not yet part of standard treatment protocol.
SC Delivery Technologies and Formulation Innovations
Advances in subcutaneous delivery technologies have significantly enhanced the precision and efficacy of cancer immunotherapy.
- Nanoparticle-based platforms, including lipid nanoparticles and polymeric carriers, allow for co-delivery of tumor antigens and adjuvants (e.g., TLR or STING agonists), improving uptake by dendritic cells and promoting robust T cell priming.
- Liposomes and microneedle arrays further optimize subcutaneous delivery by enabling targeted antigen release and minimally invasive administration. Microneedles, in particular, provide reproducible intradermal delivery with high local immune engagement and reduced discomfort.
- Biodegradable hydrogels form antigen depots at the injection site, allowing controlled release over time and sustained stimulation of antigen-presenting cells. This approach reduces dosing frequency and enhances safety by limiting systemic exposure.
- Platforms utilizing self-amplifying RNA (saRNA) and plasmid DNA—pioneered by companies like BioNTech, CureVac, and Moderna—are being tailored for SC administration. These systems drive in situ expression of tumor antigens and elicit potent cellular immunity in early-phase cancer vaccine trials.
Safety, Regulatory Considerations, and Biomarker Development
Clinical trials evaluating subcutaneous immunotherapy (SCIT) have demonstrated a favorable safety profile, with most adverse events limited to mild-to-moderate injection-site reactions, such as erythema, swelling, and transient discomfort. Systemic toxicities are infrequent, making SCIT an attractive option for repeated dosing and combination regimens.
From a regulatory perspective, EMA and FDA have shown growing interest in SC-delivered agents, including checkpoint inhibitors and cytokines formulated for subcutaneous use. Several SC versions of approved IV immunotherapies are under development or early-phase evaluation, reflecting a shift toward patient-friendly delivery models.
In parallel, biomarker discovery efforts are underway to better understand and predict SCIT efficacy. Immune profilingpost-treatment—including analysis of circulating T cells, cytokine signatures, and delayed-type hypersensitivity (DTH) responses—offers insight into systemic and localized immune activation. Additionally, T cell receptor (TCR) clonality, injection-site biopsy immunophenotyping, and expression of activation markers are being explored as potential predictive biomarkers of therapeutic response.
Future Directions and Challenges
Despite promising early results, large-scale randomized trials are needed to validate the clinical efficacy of SCIT across tumor types and treatment settings. Future development will likely focus on personalized combination regimens, integrating SCIT with radiotherapy, chemotherapy, or targeted therapies to enhance immunologic synergy.
Advances in formulation science are essential to improve antigen stability, batch consistency, and scalability, particularly for complex biologics. Additionally, the rise of personalized neoantigen-based SCIT platforms underscores the need for rapid, patient-specific vaccine manufacturing and streamlined regulatory pathways.
Importantly, SCIT’s logistical simplicity and reduced reliance on infusion infrastructure make it well-suited for low- and middle-income countries (LMICs), offering a more accessible alternative to intravenous immunotherapies. Bridging these global access gaps will be key to expanding the impact of cancer immunotherapy worldwide.
You Can Watch More on OncoDaily Youtube TV