Danish Cancer Institute shared a post on LinkedIn:
“A new infrastructure facility at the Danish Cancer Institute will give researchers from across Denmark access to brand-new, state-of-the-art technology that couples the genetic signature of a cell with a visual readout under the microscope.
The facility is funded by the Novo Nordisk Foundation and will be operational in 2025.
In the video, Jakob Nilsson — future head of the Optical Pooled Screening Facility — explains more.”
The emergence of CRISPR gene editing technology has heightened the need for comprehensive characterization of phenotypes resulting from altered genotypes. One effective method for this is optical pooled screening (OPS).
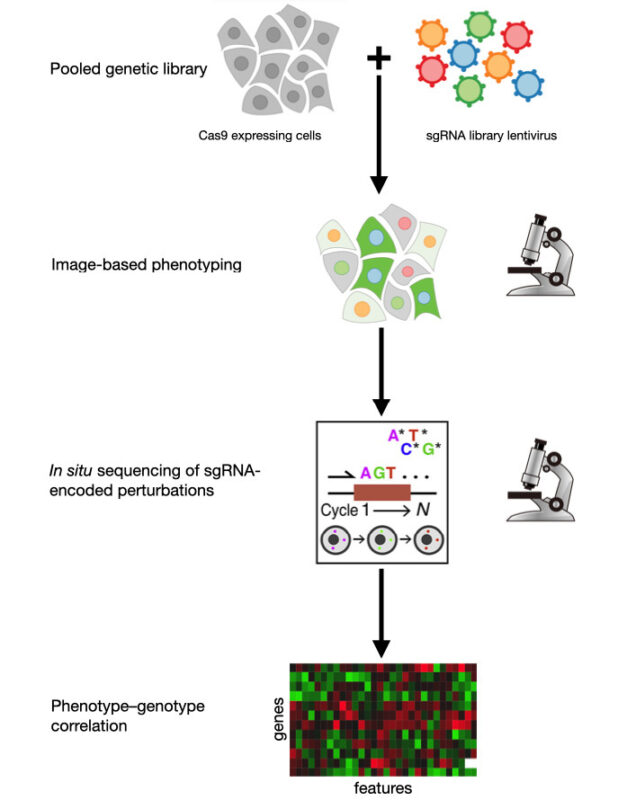
In OPS, a genetically diverse library of live cells is imaged to identify phenotypic variations without prior knowledge of the genotypes. The genotypes are later determined either in situ after the cells have been fixed or through the physical extraction of notable phenotypes followed by sequencing (Figure 1). Much of OPS development has taken place in Professor Paul Blainey’s lab at the Massachusetts Institute of Technology.
The CELESTA Light Engine is preferred for both immunofluorescence phenotyping and in situ sequencing, as its high light output reduces exposure times for in situ sequencing by 5 to 10 times compared to earlier methods using the SOLA Light Engine.
Given that in situ sequencing involves multiple cycles of dye incorporation, imaging, and cleavage, this results in significant time savings.
Recently, Blainey and colleagues enhanced OPS capabilities, enabling the analysis of 80,000 CRISPR knockout perturbations from single-cell images of around 10 million cells (3). This work has shed light on previously unexamined changes in the subcellular localization of host and viral proteins during Sendai virus infection of mammalian cells.
These advanced OPS techniques utilize the CELESTA’s optical power, spectral purity, and stability, relying on robust laser illumination for speed, diverse multiplexing, and consistency.
For more posts like this, visit oncodaily.com


