Eric K. Singhi, Thoracic Medical Oncologist and Assistant Professor at the MD Anderson Cancer Center, recently shared a post on X/Twitter:
“Great to see this option approved for our patients with Exon 20 Group insertions.
Optimizing tolerance for patients on amivantamb remains a challenge (rash can be difficult to manage and can require dermatology help).
Still eagerly awaiting data from small molecule TKIs.”
Quoting Oncology Brothers‘ post:
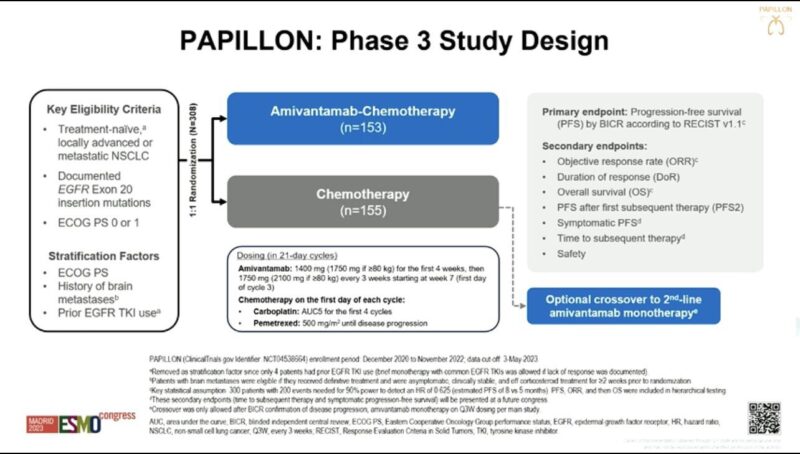
“Amivantamab now FDA Oncology approved for mNSCLC Exon20 based off PAPPILON in 1L w/ chemo:
– PFS 11.4mos w/Ami vs 6.7mos chemo (HR: 0.40)
– ORR in 73% vs 47%
– OS favoring Ami (HR: 0.67)
– 7% Ami because of AEs
– New SoC/Practice Changing”
Source: Eric K. Singhi/X


