Udhayvir Grewal, Resident Physician at Ochsner LSU Health Shreveport, recently shared a post on X:
“Hot off the press.
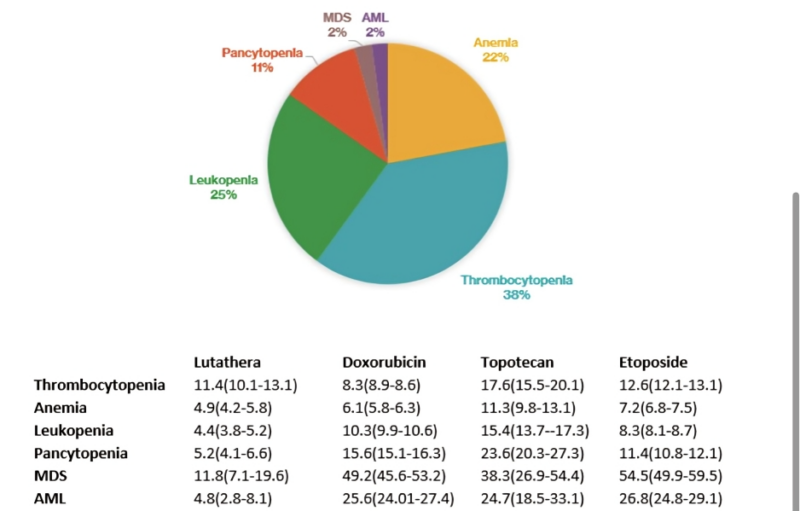
Happy to share our real-world post-marketing pharmacovigilance analysis on hematological toxicities (focus on MDS/AML) with Lutathera (Lu177-DOTA-TATE) in the US.
- Post-marketing surveillance data from the U.S. FDA (2018-2023)
- 3443 adverse events, 243 (7.1%) were hematologic
- Majority thrombocytopenia, leukopenia, anemia, and pancytopenia
- MDS and AML comprised 2% of the reported events each.
- For context, we compare the reporting of hematological toxicities (esp MDS and AML) with topoisomerase inhibitors
- This is a helpful reference that may potentially inform discussions with patients planning to receive PRRT (now referred to as radioligand therapy or RLT).
Limitations – captures only the US data and the events reported to the U.S. FDA.
Thank you Anuj Thakre for your help with the project.”
Read further
Source: Udhayvir Grewal/X


