Piotr Wysocki recently posted on LinkedIn:
“The Targeted Agent and Profiling Utilization Registry (TAPUR) Study is a prospective, phase II basket trial to identify signals of antitumor activity of available targeted agents in patients with advanced cancers harboring targetable molecular features.
In the Journal of Clinical Oncology, Cannon TL et al. have published data on the clinical activity of pertuzumab and trastuzumab combination in patients with advanced biliary tract cancer that harbored HER2/3 alterations.
The study enrolled 29 patients, of whom 45% had HER2 amplification only, 24% had HER2 mutation only, 7% had HER2 overexpression only, 10% had HER2 amplification and mutation, and 12% had other HER2/HER3 genomic alterations.
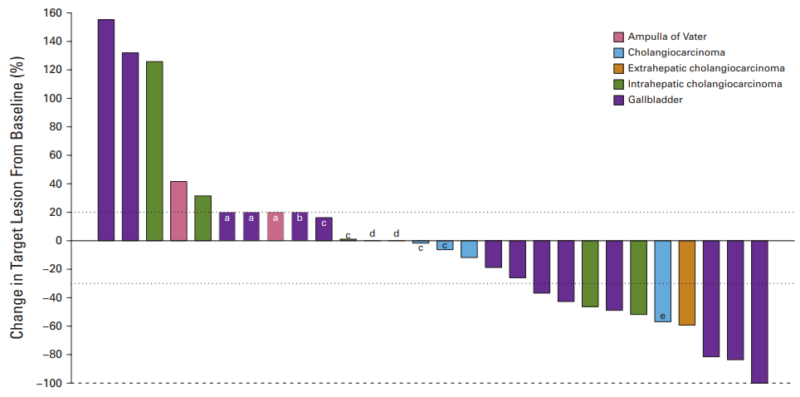
Of the 28 evaluable patients, one had CR, eight had PR, and two had SD lasting >16 weeks. The disease-control rate was 40%, and the overall response rate was 32%. The patient with CR had gallbladder cancer with a HER2 amplification and co-mutations in SMAD4 and TP53.
Of the eight patients with PR, five had gallbladder cancer, two had ICC, and one had extrahepatic cholangiocarcinoma. These eight patients had tumors with only ERBB2 amplifications, HER2 mutations (S310Y and S310F), or both HER2 amplification and a HER3 mutation.
For all patients evaluable for efficacy, the median PFS was 11 weeks (95% CI, 8 to 16), and the median OS was 30 weeks (95% CI, 17 to 49).
The analysis confirms the activity of anti-HER2 therapies in patients with biliary tract cancer harboring HER2/3 alterations and supports the use of anti-HER2 agents in clinical practice. The safety of anti-HER2 agents and the possibility of combining them with standard chemotherapy make such strategies highly feasible and affordable.”
Authors: Timothy L. Cannon, Michael Rothe, Pam K. Mangat, Elizabeth Garrett-Mayer, Vi K. Chiu, Jimmy Hwang, Namrata Vijayvergia, Olatunji B. Alese, Elie G. Dib, Herbert L. Duvivier, Kelsey A. Klute, Vaibhav Sahai, Eugene R. Ahn, Pablo Bedano, Deepti Behl, Sarah Sinclair, Ramya Thota, Walter J. Urba, Eddy S. Yang, Gina N. Grantham, Dominique C. Hinshaw, Abigail Gregory, Susan Halabi, Richard L. Schilsky
Source: Piotr Wysocki/LinkedIn
Piotr Wysocki leads the Clinical Oncology Department at University Hospital and the Faculty of Oncology at Jagiellonian University-Medical College in Krakow, Poland. As an advisor to the Polish Ministry of Health, he shapes the national cancer strategy.
His clinical expertise spans the systemic treatment of breast, gynecologic, and genitourinary cancers, with a focus on developing innovative metronomic chemotherapy-based therapies for advanced cancer patients who have undergone prior treatment.
Read other posts by Piotr Wysocki published on OncoDaily.


