Jarrod Dudakov, Associate Professor at Fred Hutch, shared on X about a recent paper by Anastasia Kousa et al. titled “Age-related epithelial defects limit thymic function and regeneration” published in Nature Immunology.
Authors: Anastasia Kousa, et al.
“With a mix of pride, enthusiasm, and tremendous relief, I present our latest paper that describes the emergence of atypical epithelial cells in the thymus with age that limit the tissues function at steady-state and its repair after damage…
This was a fantastic collaboration between Jarrod Dudakov (Fred Hutchinson Cancer Center), Marcel van den Brink/van den Brink Lab, and the twitterless Daniel Gray from WEHI (Walter and Eliza Hall Institute): with amazing assistance from Nancy Manley (Arizona State University), Manu Setty, Dana Peer, and Laura Hale from Roma.
We all know from texts like Janeway that the thymus is one of the earliest organs to show signs of aging.
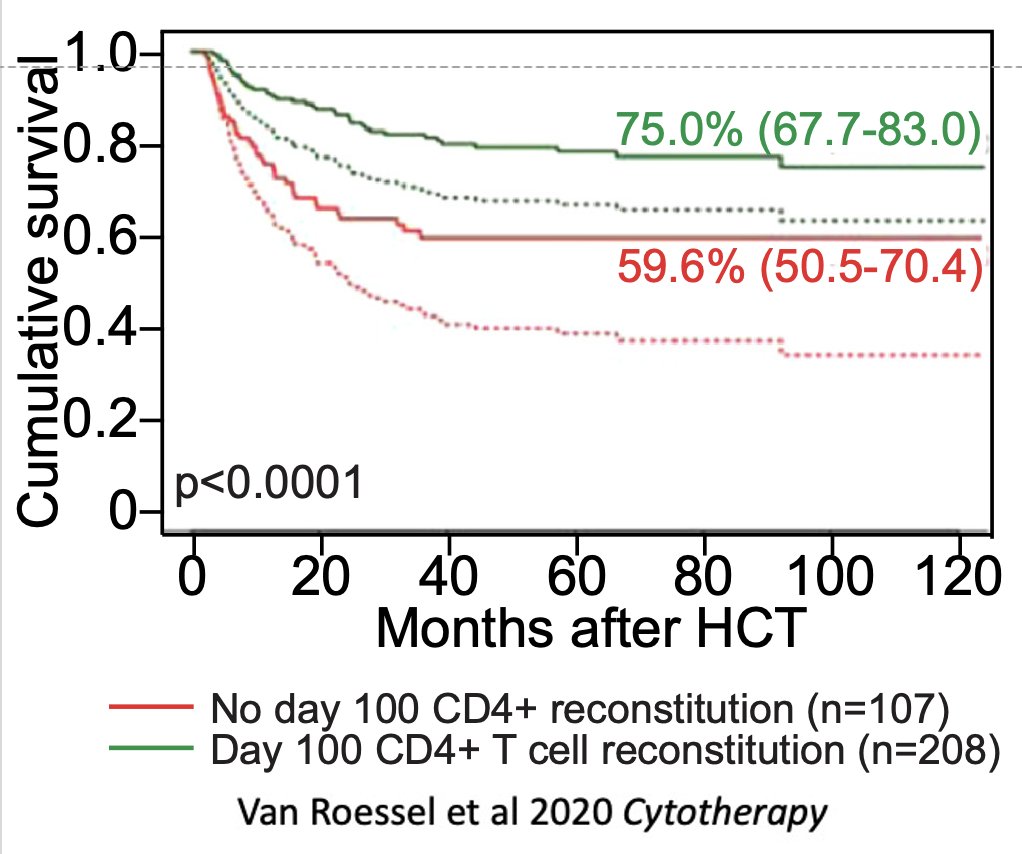
What is less well known is that this decline makes recovery from immune depleting therapies like chemotherapy and radiation more prolonged, leading to poor clinical outcomes.
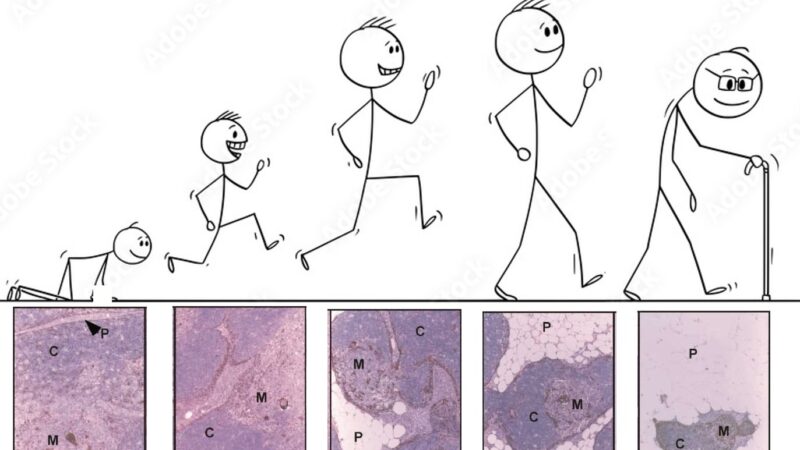
So around 10 years ago van den Brink Lab and Jarrod Dudakov set out to comprehensively understand thymic aging and damage. Starting with bulk RNA sequencing and moving on to scRNAseq, we first focused on responses of the non-hematopoietic stroma to irradiation in young and aged mice.
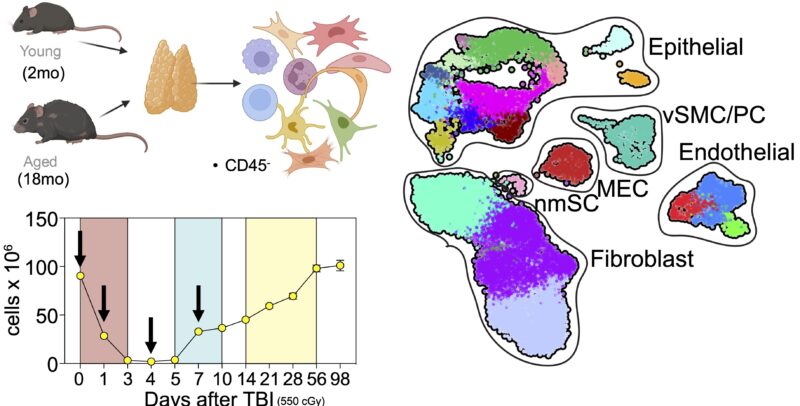
Wet lab experiments were started by Lorenz Jahn, but as anyone who has done any scRNAseq can tell you, the biggest challenge is not acquiring the data but rather interpreting it! A task made a lot easier with a tremendous shared bioinformatician: Dr. Anastasia Kousa.

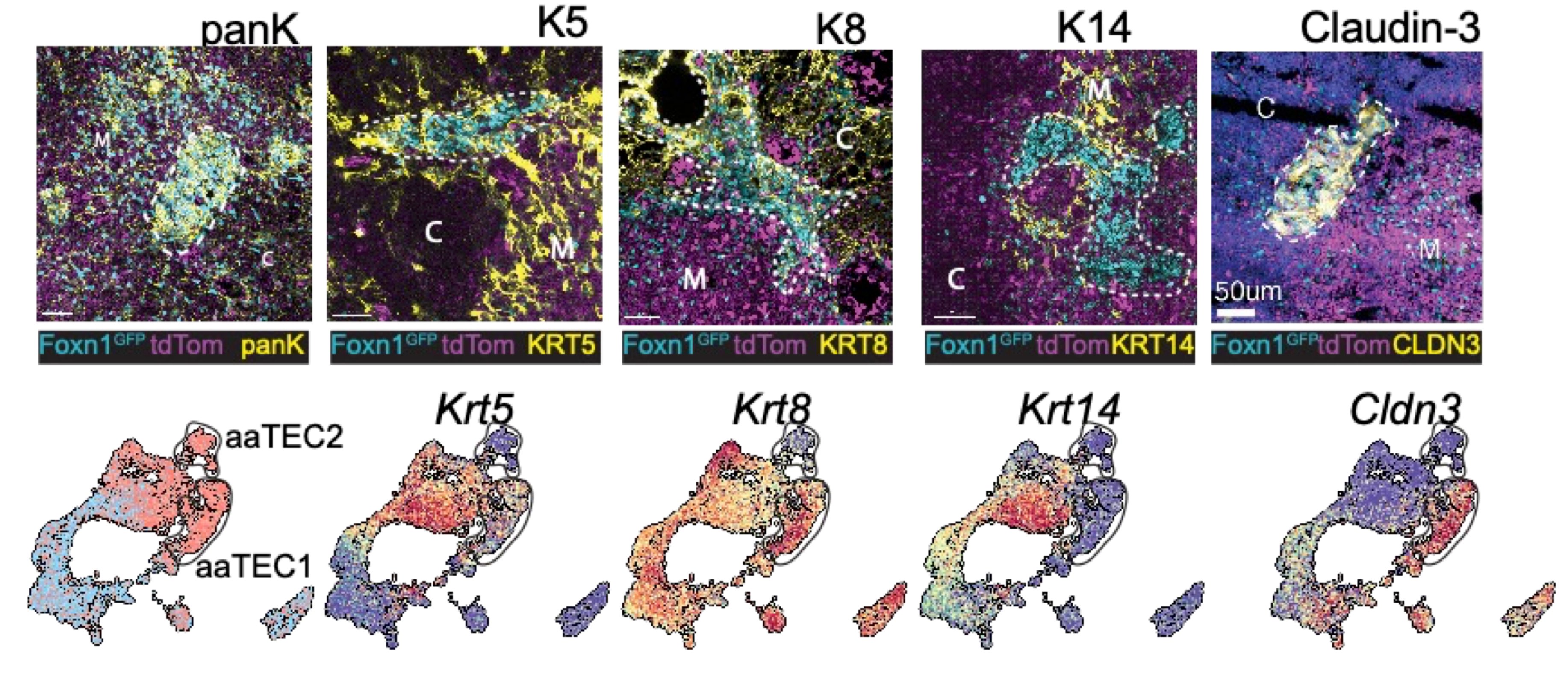
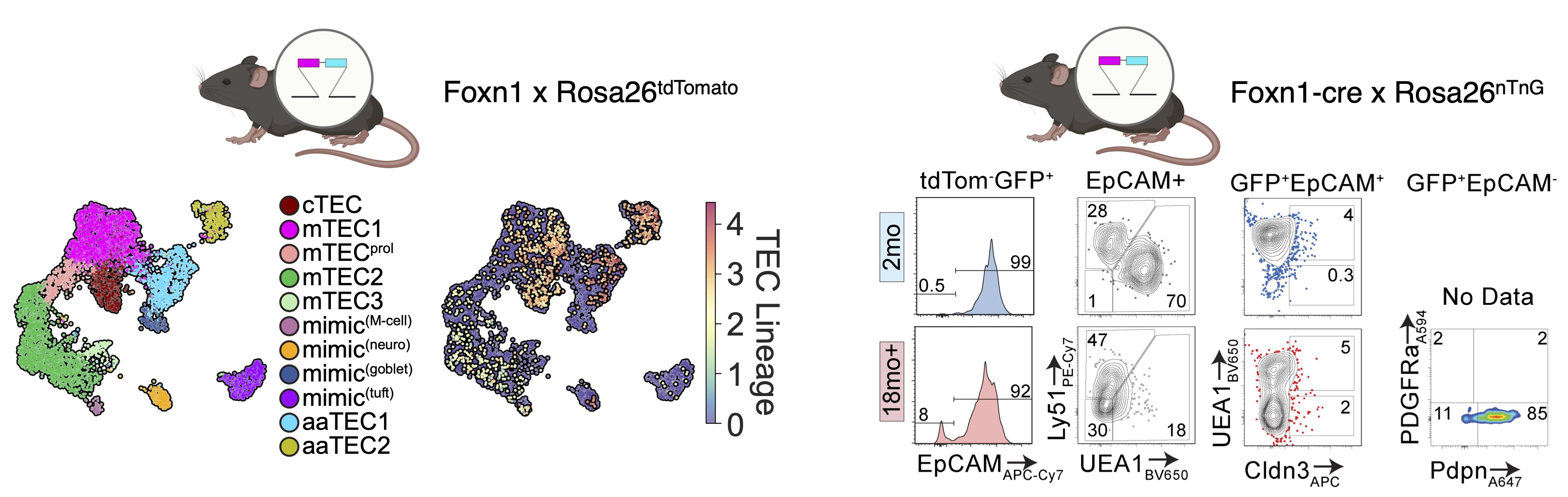
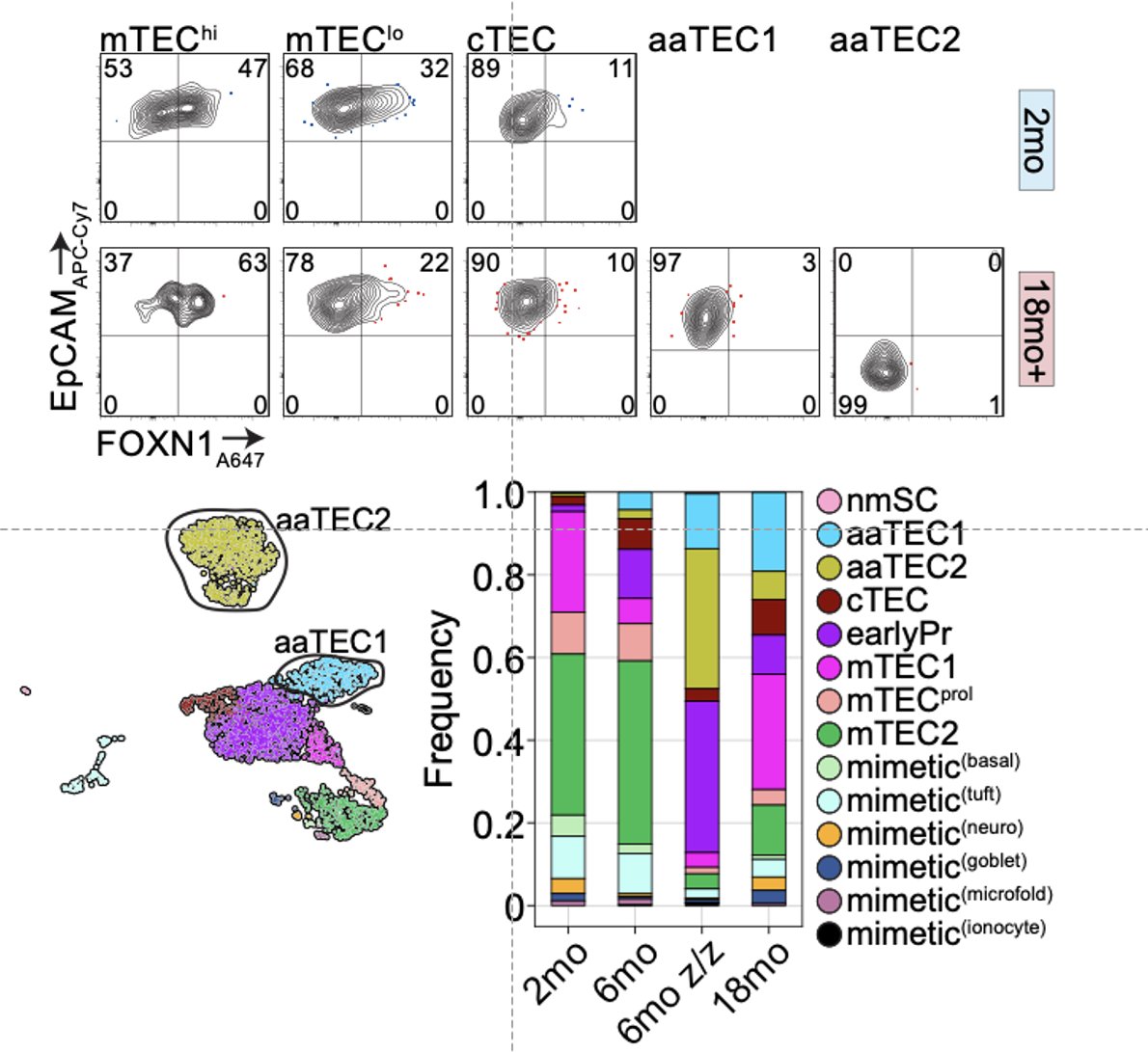
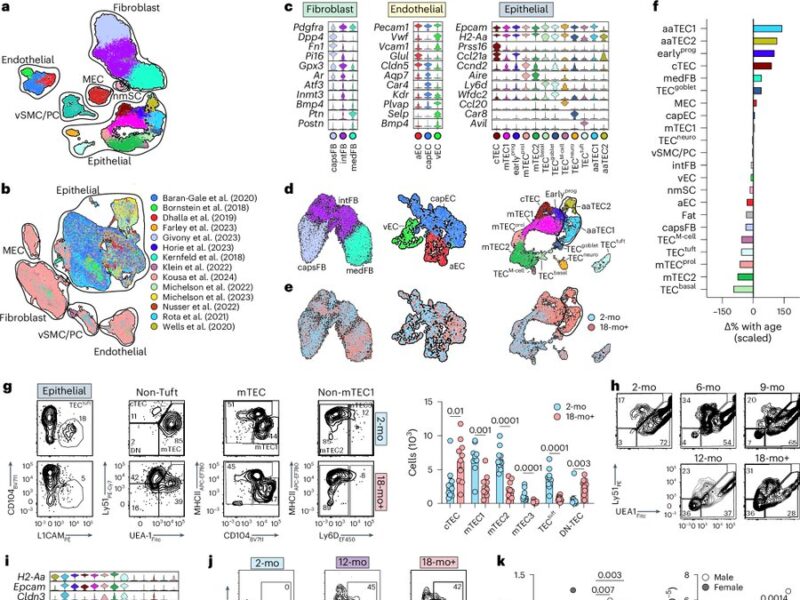
What we found is that while the fibroblast and endothelial compartments do not change at the population level with age, we observed the emergence of two age-associated TEC populations that were just not at all present in the young thymus.
We originally submitted this manuscript and uploaded a preprint with this finding but had a bit fo a hard time getting our “atlas of thymic damage” published.
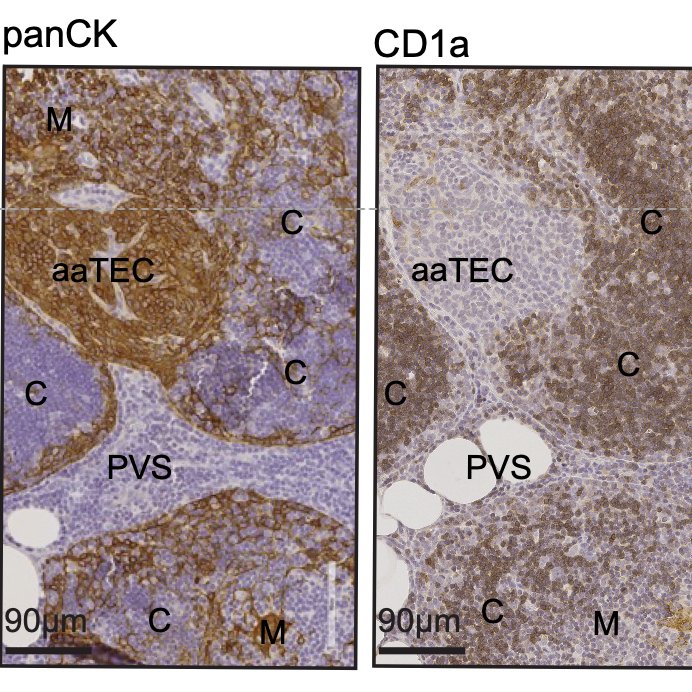
With serendipity (and testament to the power of BioRxiv) Daniel Gray reached out to say that his amazing student Kelin Zhao had been imaging the aging thymus using advanced techniques and kept finding these high-density eptihelial regions that only emerged in the aged thymus.
It is worth noting that Daniel and I crossed over in the lab of Richard Boyd Monash University and I have always had enormous respect for his science so was thrilled when this opportunity to collaborate came up.
Naturally we wanted to see if our aaTECs were the same as Dan and Kelin’s high density TEC regions, and after a huge amount of work we had determined that they were!
These regions were weirdly thymocyte deserts, suggesting they did not support T cell development. Notably, epithelial-rich regions devoid of thymocytes is also a feature of the aging human thymus, confirmed by our collaborator Laura Hale from Roma.
Now when I say these were atypical, I mean it! They had lost markers of TEC identity, and aaTEC2 had even lost much of their epithelial identity.
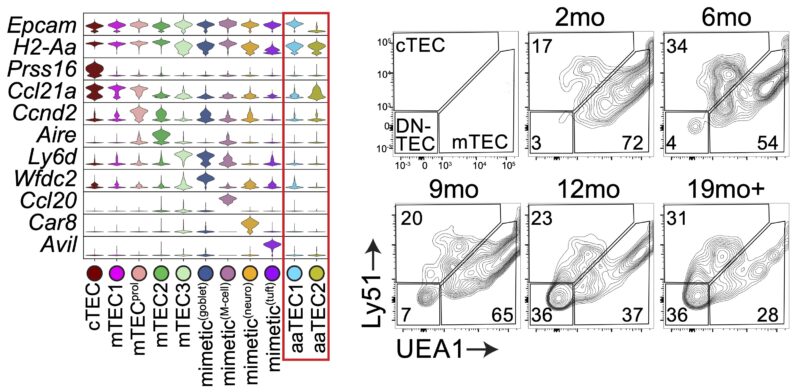
But we knew from two different lineage-tracing approaches that these aaTECs did in fact derive from FOXN1+ TECs!
Using some fancy bioinformatics tools, we found that aaTECs emerged largely from the mTEC1 population, and especially a subset with a signature matching that previously described by Thomas Boehm (link).
We also found that aaTEC emergence correlated with decreased FOXN1 expression, and were present at high frequency in mice with reduced postnatal expression and premature involution developed by our collaborator Nancy Manley (link).
Why does this matter? Thymic function is crucial, even in adults with recent work showing those who have had their thymus removed have increased cancer incidence (link) – understanding how and why the thymus ages can offer new therapeutic opportunities.
With age there is also a profound defect in the thymus’ ability to regenerate after damage, such as in patients receiving bone marrow transplant. This is key because the work of Jaap Boelens and others has shown that T cell reconstitution is a major predictor of clinical outcomes.
While aaTECs were depleted after acute damage such as that caused by ionizing radiation, they rapidly re-emerged – faster than typical TECs – suggesting a mechanism why the thymus does not repair as effectively with age.
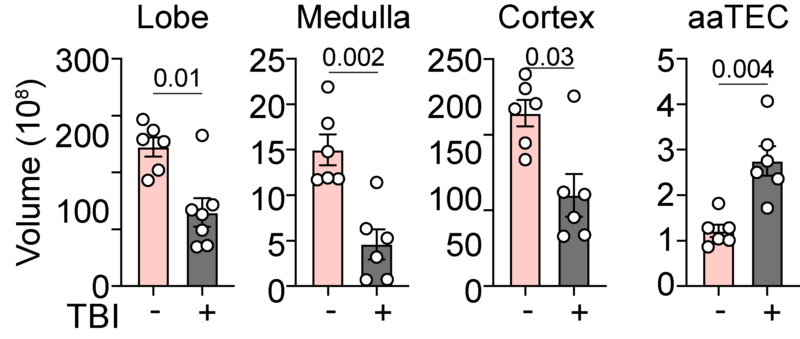
Evidence for this was provided by the sequencing data that showed that not only is the endogenous regenerative response reduced with age, but what signals do exist are ‘stolen’ by aaTECs.
So that’s our story. Emergence of age-associated TECs limit steady-state thymic function and impair its endogenous repair after damage. A lot of work now to be done to really drill into the molecular mechanisms driving aaTEC emergence and to therapeutically target them.
And of course, thanks to all our funders: NIH NHLBI, NIAID Funding, The National Institute on Aging (NIA), National Cancer Institute, NHMRC. And also a special thanks to Nature Immunology who have been excellent to work with through the publication process.”
Source: Jarrod Dudakov/X