William Schpero, assistant professor in the Division of Health Policy and Economics of the Department of Population Health Sciences at the Joan and Sanford, shared on X:
“There are long-standing disparities in clinical trial participation in the United States. In a new study in JCO, we provide evidence that Medicaid may be an important lever for addressing them.
Despite some improvements in recent years, minority patients are under-represented in trials. This affects both innovation and equity. Aaron Schwartz, Marcella Alsan, et al., have a thoughtful piece on this NEJM.
Why Diverse Clinical Trial Participation Matters
Autors:

Moreover, we have new evidence from Marcella Alsan and colleagues that equitable enrollment in trials increases patients’ interest and confidence in new therapies.
Representation and Extrapolation: Evidence from Clinical Trials
Autors: Marcella Alsan,

For decades, Medicare + private payers have covered the ‘routine costs’ of clinical trial participation – but not Medicaid. By 2022, only ~16 states mandated coverage of trial costs in their Medicaid programs. For more, see our earlier piece NEJM.
A Hidden Opportunity — Medicaid’s Role in Supporting Equitable Access to Clinical Trials
Autors:

We study these mandates in the context of the ACA’s Medicaid expansion. We ask:
- Does expansion affect Black / Hispanic patient enrollment in trials?
- Does this effect vary based on whether states have these mandates requiring coverage of the costs of participation?
We rely on data from Medidata, which has one of the largest repositories of de-identified information on US clinical trial participants. We conducted a difference-in-differences analysis limited to adults ages 18-64 enrolled in non-observational oncology trials 2012-2019.
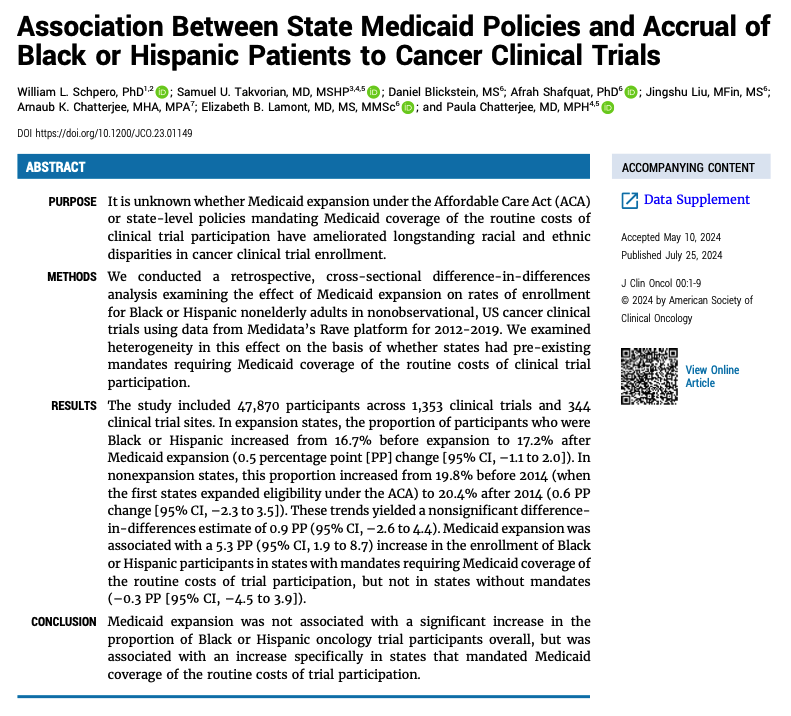
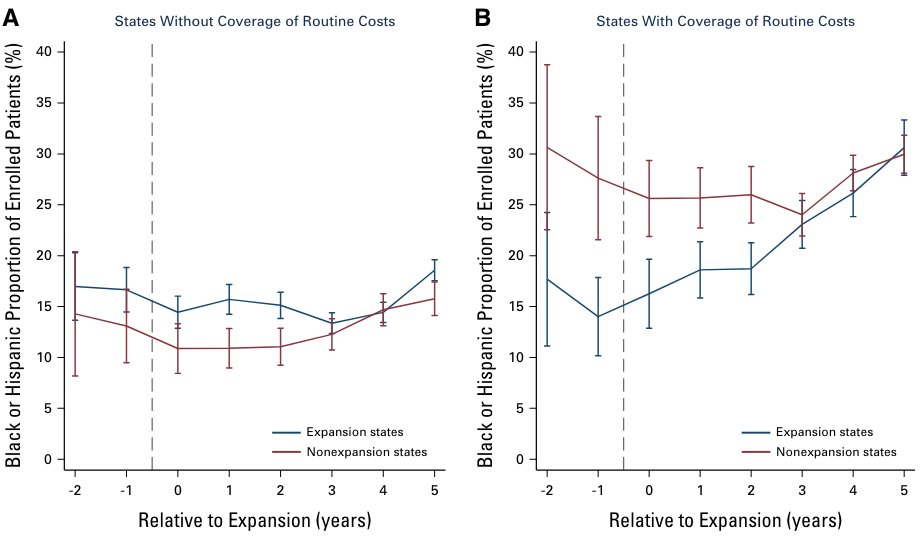
We found that ACA Medicaid expansion was not associated w/ a significant change in Black / Hispanic patient enrollment in trials. However, Medicaid expansion was associated w/ a 5 PP *increase* in states that mandated Medicaid coverage of the costs of trial participation.
This study has lots of important caveats. For example, due to data limitations, we can’t study the introduction of the coverage mandates directly. And while we rely on a large repository of clinical trial enrollment data, the data may not be fully representative.
That said, we think this is suggestive evidence Medicaid can be a policy lever for improving equity in clinical trial participation. The good news: Congress mandated the coverage of the routine costs of trial participation in Medicaid nationally beginning in 2022.
We hope our findings redouble state efforts to ensure full implementation of the federal coverage mandate — and highlight the importance of communicating this policy change to the clinicians responsible for getting patients referred to and enrolled in clinical trials.
Very grateful to the team Medidata for partnering with us in this study. And honored to co-lead this work with Sam Takvorian and Paula Chatterjee.”
Source: William Schpero/X