Hung Trinh, Director of Process Development and MFG Science and Technology at Vyriad, posted on LinkedIn:
“Trial of cell-based therapy for high-risk lymphoma leads to FDA breakthrough designation
share.
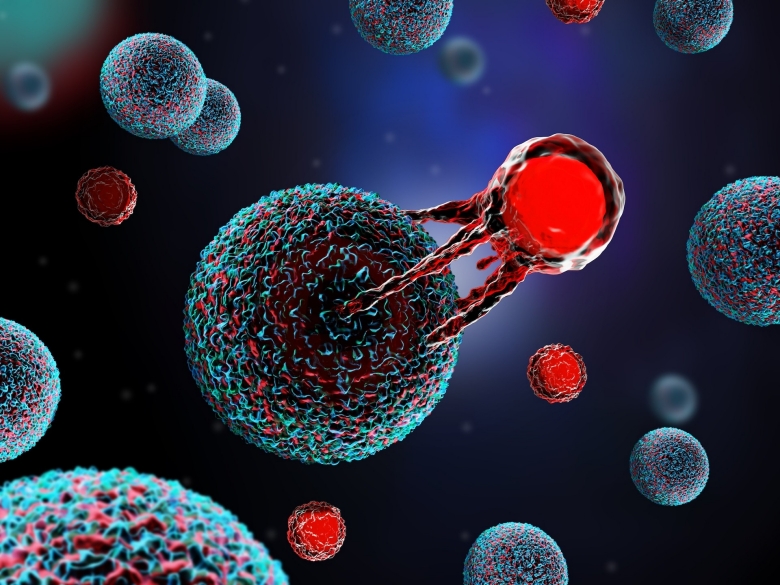
In an early Stanford Medicine study, CAR-T cell therapy helps some with intractable lymphoma, but those who relapse have few options. Modifying the therapy’s molecular target improved response.
CAR-T cell therapy, which targets a specific protein on the surface of cancer cells, causes tumors to shrink or disappear in about half of patients with large B-cell lymphoma who haven’t experienced improvement with chemotherapy treatments.
But if this CAR-T treatment fails, or the cancer returns yet again — as happens in approximately half of people — the prognosis is dire. The median survival time after relapse is about six months.
Now, a phase 1 clinical trial at Stanford Medicine has found that a new CAR-T cell therapy that targets a different protein on the surface of the cancer cells significantly improved these patients’ outcomes: Over half of 38 people enrolled in the trial — 37 of whom had already relapsed from the original CAR-T therapy — experienced a complete response of their cancers. More than half of all treated patients lived at least two years after treatment.
‘On average, the patients enrolled in this trial had received four previous lines of therapy,’ said assistant professor of medicine and the trial’s principal investigator Matthew Frank. ‘These patients are out of likely curative options, and they are scared. Half of them will die within five to six months. But in this trial, we saw a very high rate of durable complete responses, meaning their cancers became undetectable.”
Read Further.
Source: Hung Trinh/LinkedIn