On September 25, 2025, Eli Lilly and Company announced that the U.S. Food and Drug Administration (FDA) approved Inluriyo (imlunestrant), an oral estrogen receptor antagonist, for the treatment of adults with estrogen receptor–positive (ER+), HER2-negative (HER2–), ESR1-mutated advanced or metastatic breast cancer whose disease progressed after at least one line of endocrine therapy. The approval was based on results from the pivotal Phase 3 EMBER-3 trial, and the news quickly became a key milestone in metastatic breast cancer care.
EMBER-3: Key Clinical Trial Results
Endocrine resistance driven by ESR1 mutations is a major barrier in ER+, HER2– metastatic breast cancer, emerging in nearly half of patients treated with aromatase inhibitors. Imlunestrant, a next-generation oral selective estrogen receptor degrader (SERD), was designed to overcome this resistance through continuous ER blockade and degradation.
Trial Design and Methods
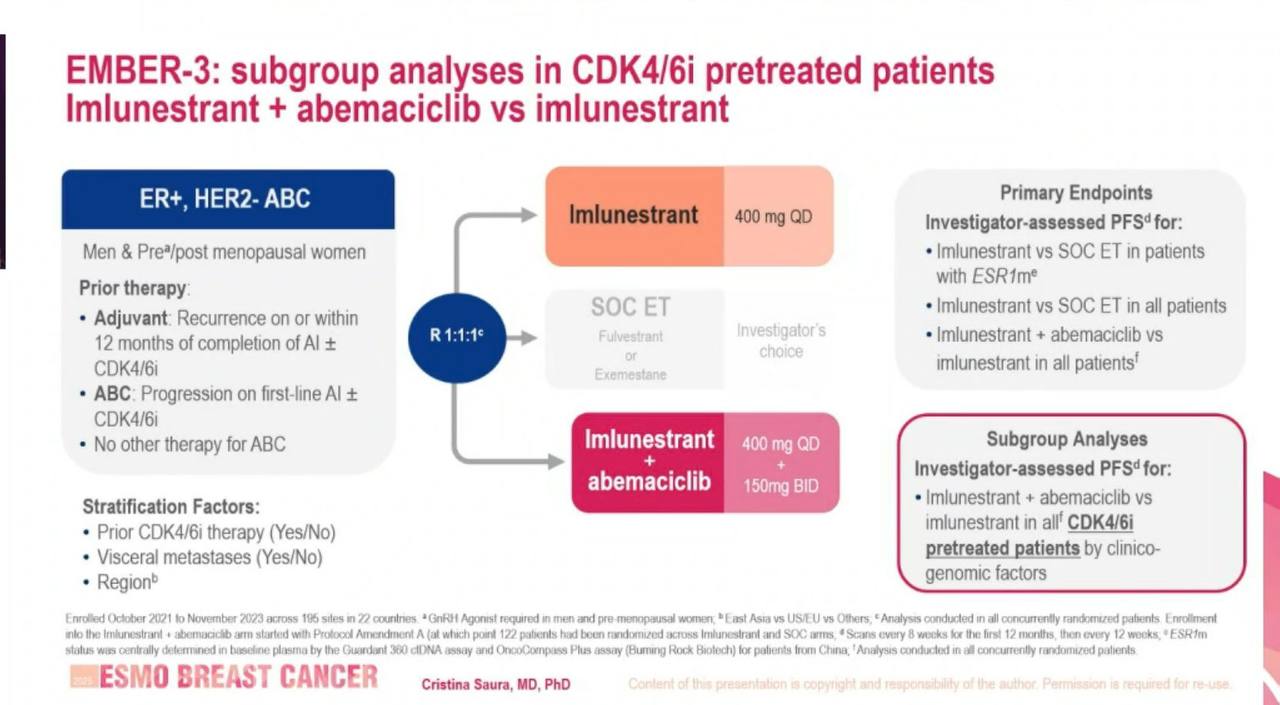
Patients with ER+, HER2– advanced or metastatic breast cancer that had recurred or progressed after aromatase inhibitor therapy (± prior CDK4/6 inhibitor) were randomized to one of three arms:
- Imlunestrant monotherapy (400 mg daily)
- Standard endocrine therapy (fulvestrant or exemestane)
- Imlunestrant + abemaciclib (150 mg BID)
Key Results
ESR1-mutated subgroup (n=256)
- Median PFS: 5.5 vs 3.8 months (HR 0.62, 95% CI 0.46–0.82; p=0.0008)
- 38% reduction in risk of progression or death
Combination arm: Median PFS: 9.4 vs 5.5 months (HR 0.57; p<0.001)
Most adverse events were low-grade, with <5% discontinuing imlunestrant monotherapy.
These findings established imlunestrant as an effective oral SERD for patients with ESR1-mutated disease, while the combination with abemaciclib showed added benefit across the broader ER+/HER2– population.
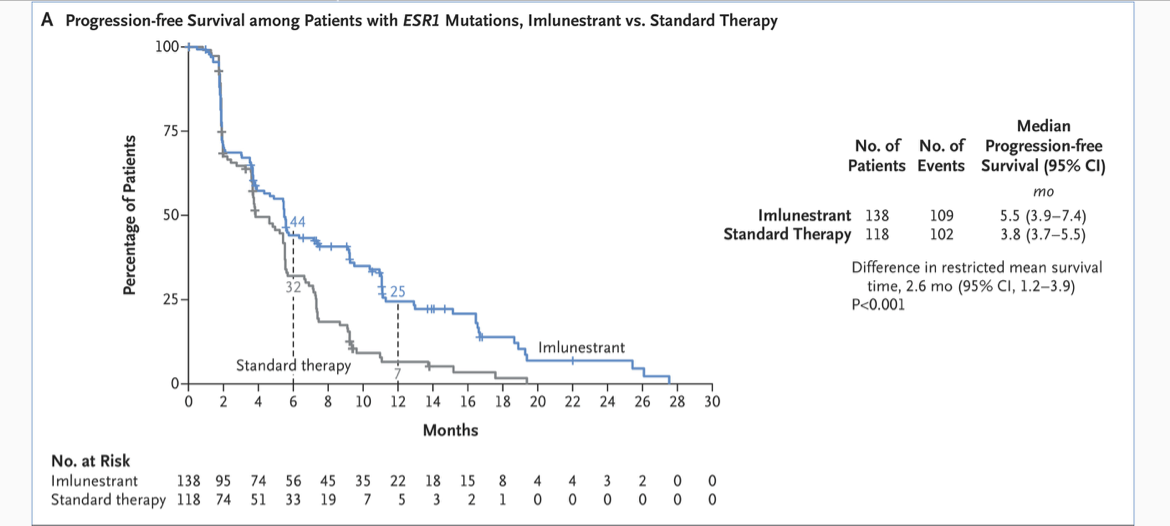
Figure From Original Article: Investigator-Assessed Progression-free Survival with Imlunestrant as Compared with Standard Therapy
What drug is Imlunestrant?
Imlunestrant (Inluriyo) is a next-generation oral estrogen receptor antagonist. It binds to and blocks estrogen receptors while also promoting their degradation, including receptors harboring ESR1 mutations. These mutations—acquired during or after treatment with aromatase inhibitors—occur in about 50% of patients with ER+, HER2– metastatic breast cancer and are a major driver of treatment resistance. By overcoming this mechanism, imlunestrant offers patients an all-oral therapy option with demonstrated clinical benefit.
Who Manufactures Inluriyo?
Inluriyo is developed and manufactured by Eli Lilly and Company (NYSE: LLY), a global pharmaceutical company headquartered in Indianapolis. Lilly is currently studying imlunestrant in additional settings, including the Phase 3 EMBER-4 trial in early breast cancer, enrolling approximately 8,000 patients worldwide.
You Can Read More On Ember-3 Trial Data Presented at ESMO Breast 2025 on OncoDaily
What is Next?
The FDA approval of imlunestrant (Inluriyo) marks a significant advance for patients with ESR1-mutated, ER+/HER2– metastatic breast cancer, a population with few effective options after endocrine resistance develops. By offering an oral, targeted therapy that directly addresses a key resistance mechanism, this milestone expands precision treatment choices and reinforces the importance of routine ESR1 mutation testing in clinical practice.
With the ongoing EMBER-4 trial now evaluating imlunestrant in early breast cancer, further data will clarify its role across the disease spectrum. For now, this approval not only broadens the therapeutic landscape but also signals a new era of mutation-directed endocrine therapy in breast cancer.